안녕하세요 보스턴 임박사입니다.
MASH (NASH)는 간질환 중에서 가장 환자들도 많고 시장도 그만큼 큽니다. 저도 오랜기간 MASH 치료제 개발에 관심을 가지고 있고 Madrigal의 Resmetirom에 대해 얘기를 한 적이 있습니다.
- MASH: Metabolic dysfunction-Associated SteatoHepatitis
- NASH: Non-Alcoholic SteatoHepatitis
BIOTECH (10) – Madrigal Pharmaceuticals의 NASH 치료제 MGL-3196 (Resmetirom) 임상 3상 결과
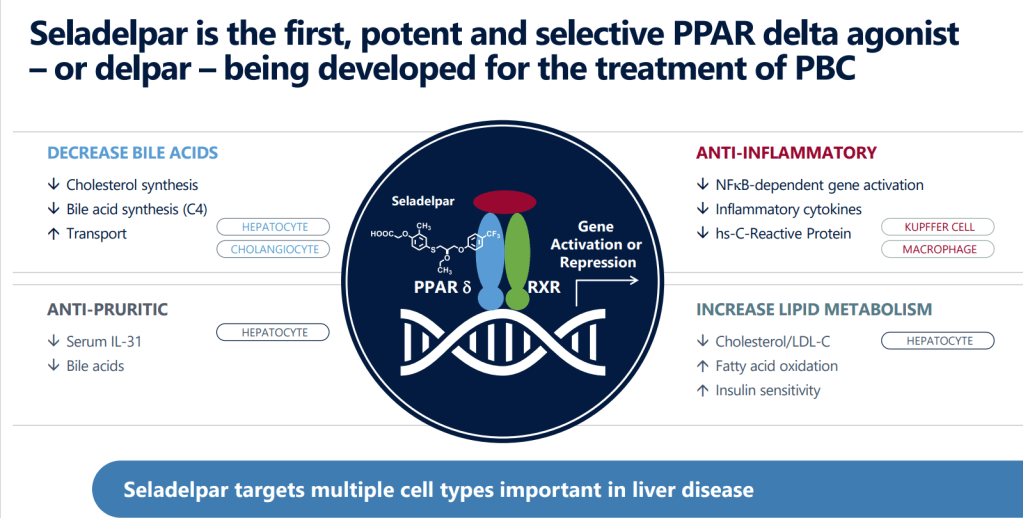
CymaBay Therapeutics는 1992년에 Metabolex라는 회사명으로 설립해서 PPAR-γ modulator 인 Arhalofenate (MBX-102)를 자체개발해서 Johnson & Johnson과 제휴로 임상시험을 하고 있었고 Janssen Pharmaceutica NV (Johnson & Johnson company)로 부터 PPAR-δ Agonist인 Seladelpar (MBX-8025, RWJ-800025) in-licensed the patents and the exclusive development and commercialization rights해서 2007년에 Dyslipidemia 치료제로 임상2상을 시작했습니다.
PPAR-targeted drugs의 최근 MASH와 연관된 NAFLD 임상시험 상황은 아래 리뷰로 볼 수 있습니다.
Metabolex Privately Raises $27M For MBX-102 Phase II Program – BioWorld 8/28/2003
Alta Bets on Resurrected Metabolex – Buyouts 12/6/2004
Metabolex, Inc. announced today that it has entered into a comprehensive global strategic agreement with Ortho-McNeil, Inc., a Johnson & Johnson (NYSE: JNJ – News) company, and will collaborate on the development and commercialization of multiple programs addressing metabolic diseases, including type 2 diabetes, obesity and dyslipidemia. The agreement includes Metabolex’s two clinical compounds, metaglidasen and MBX-2044, and additional follow-on compounds for which Ortho-McNeil has received an exclusive license for worldwide development and commercialization.
In addition, as part of the transaction, Metabolex gains exclusive license to a PPAR-delta agonist program, including RWJ-800025, which has completed Phase 1b clinical testing, and a cannabinoid receptor-1 inverse agonist clinical program, which targets obesity. This agreement also includes a target collaboration in which Ortho-McNeil and Metabolex will work together to screen metabolic disease targets identified by Metabolex.
Metabolex Begins Phase 2 Trial of MBX-8025 for Treatment of Dyslipidemia – Pipeline Review 9/26/2007
Metabolex는 2013년에 CymaBay Therapeutics로 사명을 변경하면서 증자와 함께 구조조정을 단행했습니다. PPAR-γ modulator 인 Arhalofenate (MBX-102)가 3개의 임상 파이프라인 중 대표적인 약물이었는데 Gout 치료제로서 당시 Phase 2에 있었습니다.
CymaBay는 지난주에 Seladelpar의 NDA가 FDA에 의해 접수되고 Priority Review Date가 2024년 8월 14일로 결정되었다고 발표를 했고 이날 Gilead는 CymaBay Therapeutics를 $4.3 Billion에 인수한다고 발표했습니다.
The FDA has granted priority review and set a Prescription Drug User Fee Act (PDUFA) target action date of August 14, 2024 and notified the company that it is not currently planning to hold an advisory committee meeting to discuss the application.
Gilead to Acquire CymaBay for $4.3B, Adding to Liver Portfolio – GEN Edge 2/12/2024
In September, CymaBay announced that seladapar generated positive data in the pivotal Phase III RESPONSE trial (NCT04620733), by achieving statistical significance over placebo across primary composite endpoints of:
- Biochemical response (61.7% for patients on seladelpar vs. 20.0% for placebo).
- Normalization of alkaline phosphatase (ALP) at 12 months (25.0% for patients on seladelpar vs. 0.0% for placebo).
- Improvement in pruritus at six months among people living with moderate-to-severe itch, a result that was sustained through 12 months.
Seladelpar previously received the FDA’s Breakthrough Therapy Designation, the European Medicines Agency (EMA)’s PRIME status (EMA), and the orphan drug designations of both regulatory agencies.
Seladelpar is projected to generate sales of $1.9 billion by 2029, according to the London Stock Exchange Group (LSEG), though Jefferies equity analyst Michael J. Yee has estimated the drug “could bring in $500M to $1B+ in peak sales.”
Seladelpar의 임상시험은 2019년 11월에 FDA Clinical Hold를 맞으면서 큰 위기를 겪었습니다. 한달 후 60% 임직원을 구조조정했고 8개월간의 천신만고 끝에 Clinical Hold는 해소됩니다.
CymaBay to cut 60% of workforce; shares down 8% after hours – Seeking Alpha 12/19/2019
CymaBay resurrects seladelpar 8 months after NASH flop – Fierce Biotech 7/23/2020
The development halt came after biopsies of some 30% of patients in a Phase IIb NASH/MASH trial showed atypical histological findings in the form of an interface hepatitis presentation, with or without biliary injury. That led to an FDA clinical halt, followed by CymaBay eliminating some 60% of its workforce in December 2019 and launching a strategic review of its options.
“Management was forced to make difficult operational decisions following the emergence of atypical histological findings from the Phase II nonalcoholic steatohepatitis study. We commend management’s resilience and persistence that ultimately led to seladelpar demonstrating the best-in-class clinical profile in PBC, and we expect the drug to benefit thousands of patients living with PBC as soon as later this year,” Hsieh wrote in a research note.
Seladelpar (MBX-8025)의 Patent United States Patent 9,381,181에 의해 특허유효기간은 적어도 2035년까지 유지됩니다.
2024년 1월에 발표한 CymaBay Therapeutics의 Presentation는 아래와 같습니다. Seladelpar가 FDA에서 승인되어 MASH 환자들에게 큰 도움이 되길 바랍니다.
