안녕하세요 보스턴 임박사입니다.
mRNA-based cancer vaccine or individualized neoantigen therapy는 현재 많은 Biotech에서 개발하고 있는 상황이고 특히 Moderna의 INT therapy는 아주 좋은 결과를 나타냈습니다.
Duke University의 Herbert Kim Lyerly 교수 (picture)와 Zachary Hartman 박사는 Self-Replicating mRNA cancer vaccine연구를 진행하고 있었습니다.

Duke OTC Startup – Replicate Bioscience: Replicate Bioscience is developing RNA-based treatments for cancer using proprietary Synthetic Replicon for gene delivery (SynRGY) technology to deliver the genetic code of a virally derived RNA molecule.
How close are we to a cancer vaccine? Duke University scientists are closing in – Fox8 4/11/2022
For the last several years, researchers have been working with technology based around mRNA—messenger RNA, which is something that compliments the work of your DNA—to see if they can utilize it to get your body’s own immune system to fight cancers. At Duke University, they’re working with mRNA technology to create vaccines for cancer.
“It is a product which is RNA nucleic acid which encodes a specific protein and then that can be encapsulated in something we like to call a lipid nanoparticle, which is really a little fat bubble, and that can be injected into your body and sort of teaches your body what to go after immunologically,” said Zachary Hartman who works in the Lyerly Lab at Duke.
Lyerly has been at Duke nearly 40 years and has seen massive changes in how we can combat cancer going from relatively crude chemotherapy to very targeted immunotherapy.
그러던 중 Apple Tree Partners의 Mark Ehlers는 Novartis 등에서 synthetic biology 연구를 했던 Nathaniel Wang과 mRNA vaccine development를 했던 Andrew Geall을 만나면서 Duke University의 Hebert Kim Lyerly교수와 Zachary Hartman 교수와 함께 Replicate Bioscience를 만들게 됩니다.
아래는 Andrew Geall이 2012년에 Novartis에서 발표한 PNAS논문입니다.
To Ehlers and later Apple Tree, one of the big draws to Replicate was the team. Wang was experienced at developing and applying synthetic biology after working with companies like Synthetic Genomics, Novartis and Johnson & Johnson. Andrew Geall, another co-founder, led messenger RNA vaccine development at Novartis before heading to Avidity Biosciences, where he served as vice president of formulations, analytics and chemistry. Avidity went public in June 2020, raising just shy of $260 million.
Replicate’s two other co-founders, Hebert Kim Lyerly and Zachary Hartman, are professors of cancer research and immunology at Duke University.
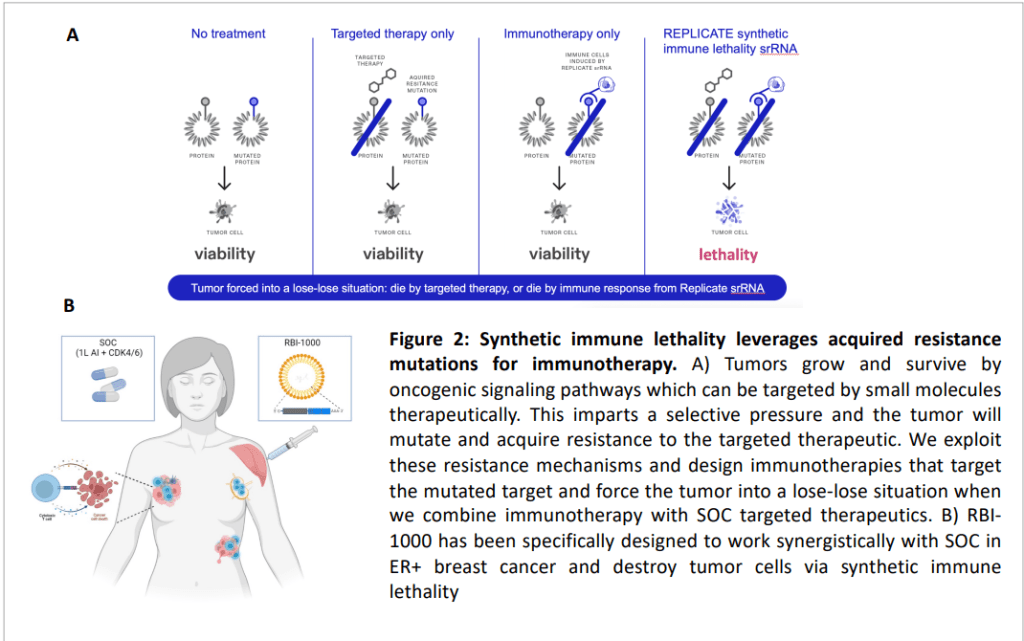
The srRNA technology is meant to prevent or remove drug-resistant cancer mutations via “synthetic immune lethality” and is also being tested in autoimmune and inflammatory disorders.
Replicate has two programs focused on breast cancer and lung cancer, another looking at immunotherapy resistance in solid tumors and a fourth program that addresses inflammation related to autoimmune disorders that goes after “downstream mediators of the inflammasome,” Wang said.
Wang was previously head of the RNA medicines unit at Synthetic Genomics, where he worked on infectious diseases and various immunotherapies. While there, he worked on collaborations with Novartis on synthetic genomics for vaccines and with Johnson & Johnson’s Janssen on replicating RNA for treating infectious diseases.
During his collaboration with Novartis, Wang met Replicate co-founder and Chief Development Andrew Geall, Ph.D., who led mRNA vaccine exploration at Novartis. Since leaving Novartis in 2015, Geall has been vice president of formulations and chemistry at Avidity Biosciences and chief scientific officer of Precision NanoSystems.
Geall and Wang teamed up with Duke University cancer research and immunology professors Herbert Kim Lyerly, M.D., and Zachary Hartman, Ph.D., to found Replicate.
Replicate Launches with $40 Million to Eliminate Drug Resistance in Cancer – Biospace 9/8/2021
Replicate focuses on using self-replicating RNA (srRNA) to develop therapies for autoimmune and inflammatory diseases and prevent drug resistance in cancer.
Replicate, which was founded in February 2020, currently has four pipeline programs, all in the discovery or preclinical phase. These include RBI-1000, an endocrine therapy resistance for breast cancer; RBI-2000, immunotherapy resistance for solid tumors; RBI-3000, targeted therapy resistance for lung cancer; and RBI-8000, inflammasome for inflammatory disease and autoimmune disorders. The first therapy is expected to begin clinical trials in the second half of 2022.
Nathan Wang, Replicate’s Chief Executive Officer and Andrew Geall, Chief Development Officer, collaborated earlier on srRNA technologies at Synthetic Genomics and at Novartis Vaccines & Diagnostics. They worked with a former colleague, Herbert Kim Lyerly, and Zachary Hartman, both professors of cancer research and immunology at Duke University, to work on srRNA to prevent or remove drug-resistant cancer mutations. This approach is called “synthetic immune lethality.”
Lyerly교수와 Hartman 교수는 2019년 Clinical Cancer Research에 Vaccine-induced memory CD8+ T Cells이 HER2-breast cancer 치료제로 가능성이 있슴을 Mouse to Human Translational Study로 발표했습니다.
Nathan Wang은 Replicate의 VP of Research로 채용된 Aliahmad와 2018년에 self-replicating RNA vectors에 대한 리뷰를 낸 바 있습니다.
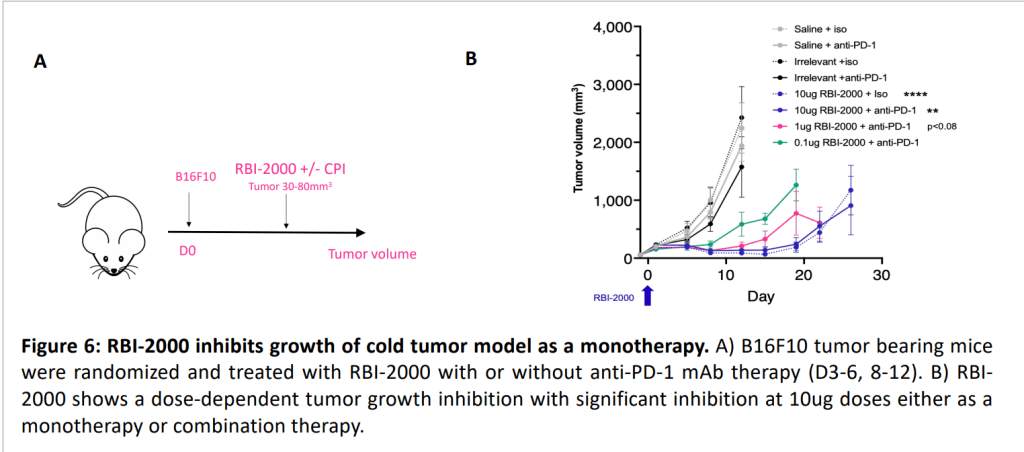
Replicate Bioscience는 2023년 4월 AACR에서 2개의 포스터를 발표했는데요 RBI-2000 cancer vaccine의 경우에는 Molecule 1 (Immune cell activator)와 Molecule 2 (reverses inflammation and prevents angiogenesis and tumor invasiveness)의 두가지 self-replicating mRNAs를 포함한다고 발표했고 Mouse PoC data를 발표했습니다.

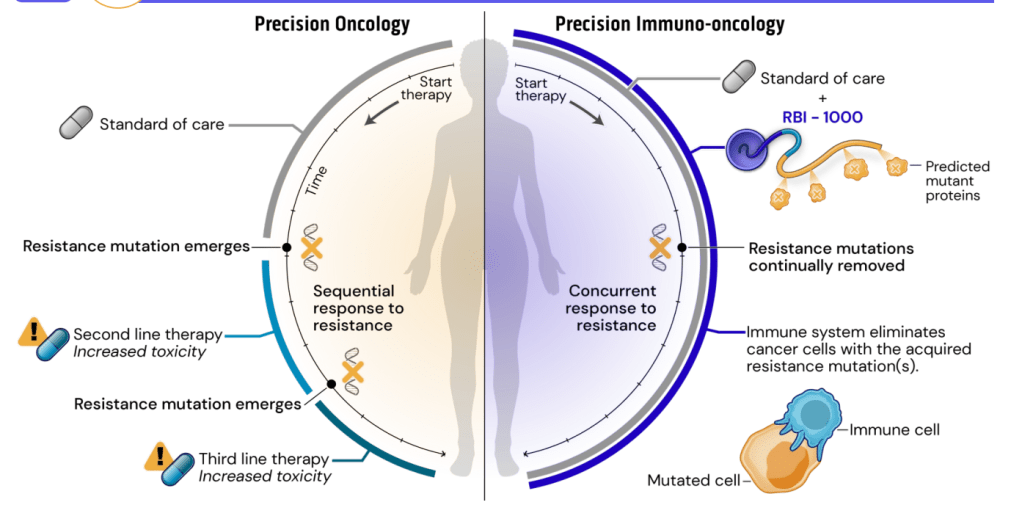
같은 AACR의 다른 포스터에서는 Self-replicating RNA로 ER+ breast cancer에 작용하는 것을 발표했습니다.
Synthetic immunology lethality concept을 아래와 같이 보여주었는데요. Chemo-reistant neoantigen을 사멸시키며 (lethality) 역시 Molecule 1과 Molecule 2를 사용합니다.
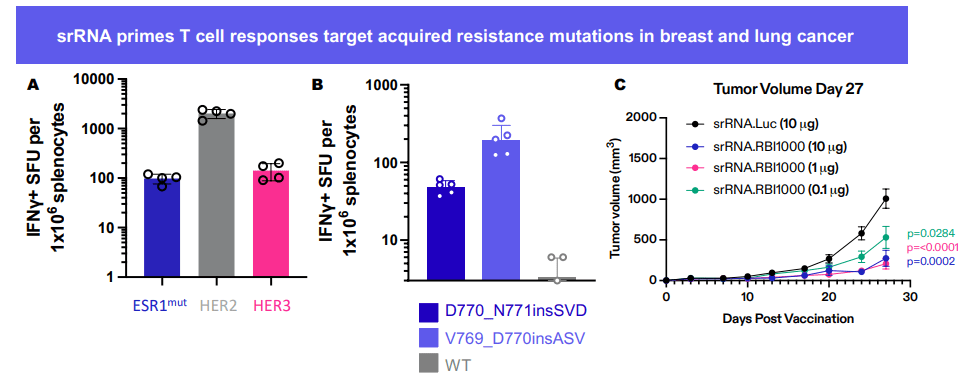
RBI-1000의 Mouse PoC data를 보여주었습니다.
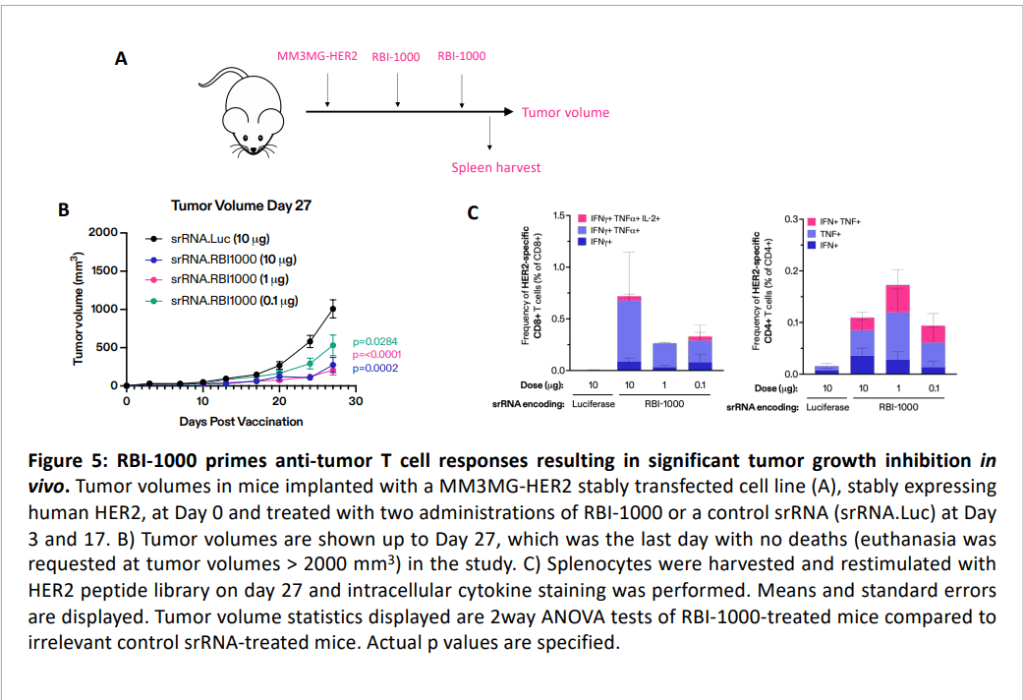
그리고 11월에 SITC학회에서 새로운 포스터를 발표했습니다.
역시 RBI-1000에 대한 것으로 breast cancer와 lung cancer에 대해 작용함을 보여주었습니다.
RBI-4000 (Rabies vaccine)에 대한 결과를 이번주에 발표했습니다.
The phase 1 trial is comparing three doses of its vaccine candidate, RBI-4000, to Bavarian Nordic’s approved product RabAvert in healthy volunteers.
In “most subjects,” all three RBI-4000 doses generated antibody titers of at least 0.5 IU/ml, the level that the World Health Organization considers to be protective. Replicate said the 0.1-mcg dose is the smallest amount of any RNA technology reported to achieve the surrogate of protection in humans.
The biotech said a single shot of RBI-4000 met the surrogate protection metric “for a majority of subjects in multiple cohorts.” Replicate tested single shots at all dose levels, plus a two-administration regimen at the highest, 10 mcg, dose. GSK studied one- and two-dose regimens of its self-amplifying mRNA rabies vaccine in a phase 1 study that finished in 2022.
Replicate saw “favorable tolerability across all dose levels tested, with no severe adverse events,” but is yet to share safety data.
Beyond rabies, Replicate CEO Nathaniel Wang, Ph.D., believes the results de-risk the srRNA platform, manufacturing processes and pipeline, boosting the biotech’s prospects as it pursues diseases including breast and lung cancers. CSL Seqirus and Arcturus Therapeutics won approval for a self-amplifying vaccine that uses similar technology last year, but Replicate is still validating its platform.
RBI-4000으로 biology risk를 낮춘 약물로서 임상시험을 하면서 srRNA platform을 검증하고 추후에 cancer vaccine trial을 준비한다는 계획입니다. saRNA or srRNA는 현재 CSL Sqirus/Arcturus Therapeutics에서 FDA 승인을 받은 상태인데 향후 Replicate의 cancer vaccine RBI-1000의 임상시험 진입과 결과가 주목됩니다.
