안녕하세요 보스턴 임박사입니다.
RAPT Therapeutics는 2015년에 Bristol-Myers Squibb (BMS)가 Flexus Biosciences를 $1.25 Billion에 인수한 이후 BMS가 관심이 없는 “FLX925, a dual inhibitor of FLT3 and CDK4/6″을 가지고 Spin off해서 FLX Bio라는 이름으로 설립되었습니다.
After San Carlos, Calif.-based Flexus Biosciences was bought by New York’s Bristol-Myers Squibb Company for $1.25 billion in February 2015, company execs Terry Rosen and Juan Jaen turned around and started a new company.
Less than two months later, two of the Flexus’s executives and founders, Terry Rosen, Flexus chief executive officer, and Juan Jaen, head of Flexus research and development, have joined to form a new company. They plan to crank out a new immuno-oncology drug candidate once a year.
The Bristol-Myers Squibb deal bought the company and the ID01 and TD02 compounds, but the rest of the assets are being spun into the new company. Although details are still pending, Rosen and Jaen expect funding will come from investors who invested in Flexus.
The most likely candidate for the new company is FLX925, a dual inhibitor of FLT3 and CDK4/6, which were licensed from Thousand Oaks, Calif.-based Amgen in 2004. They first met while students at the University of Michigan. Rosen stayed with Amgen in May 2014.
The two men worked together at South San Francisco, Calif.-based Tularik before that company was acquired by Amgen . Rosen stayed with Amgen, but Jaen left to be the chief science officer and senior vice president of drug discovery at ChemoCentryx, Inc. , then joined together in 2013 to form Flexus.
“We’ve done some good initial work,” says Rosen in a statement, “and we feel good about it, but our goal is to build something that is long term and sustainable.”
FLX Bio는 Celgene 주도하에 $29 Million Series A를 한 후 $50 Million Series B를 했습니다.
FLX’s FLX925 is a selective inhibitor of FLT3 and CDK4/6 and is being studied in a proof-of-concept study in patients with acute myeloid leukemia. The drug is moving into a Phase I clinical trial. FLX925’s dual-inhibitor action may be more effective in treating FLT3-mutated AML than other FLT3 inhibitors, resulting in greater clinical benefit. Furthermore, FLX925 has the potential to treat a wider array of cancers beyond AML, the company said on its website. In addition to FLX925, FLX also has two pre-clinical immuno-oncology programs in early development.
The $50 million raised in the latest round will go nicely with $29 million in Series A funding privately-held FLX previously raised. That makes $79 million in less than one year since FLX Bio was founded. The Series B financing was supported by multiple venture capital and biotech groups, including The Column Group (TCG), Topspin Partners, Kleiner Perkins Caufield & Byers (KPCB) and Celgene .
그리고 1년만에 다시 $60 Million Series C를 했습니다. 이 때에는 Google Ventures (GV)가 투자를 했습니다. 2017년에 CCR4 Inhibitor Tivumecirnon (FLX475)의 임상1상이 시작했습니다. BMS/Flexus Biosciences에서 가져온 FLX925는 파이프라인에서 제외되었습니다.
Celgene, GV-backed FLX gets off $60M round, kick-starts I-O trials – Fierce Biotech 12/21/2017
South San Francisco, California-based FLX emerged from Bristol-Myers Squibb’s $800 million takeover of Flexus Biosciences, with a pipeline of prospects the Big Pharma decided it could live without.
It now has the cash and the early data to push on with phase 1 tests, just started, for its oral CCR4 inhibitor FLX475. This drug focuses on a key pathway by which tumors recruit regulatory T cells to suppress tumor immunity. By blocking this pathway with a CCR4 antagonist, the biotech is betting that it can prevent regulatory T cell recruitment to the tumor site, reduce the number of regulatory T cells in the tumor and importantly, enable immune activation and tumor killing. The biotech also sees FLX475 as having the potential to boost cell-based immunotherapies, such as CAR-T and cancer vaccines, which could lead to combos in the future.
The company adds that it’s also now looking to pick out another clinical candidate next year, this time targeting ubiquitin specific protease 7 (USP7), as well as working on its GCN2 program. USP7 plays a key role in two cancer pathways: it promotes the formation and function of regulatory T cells by deubiquitinating and stabilizing FOXP3; and it maintains low levels of p53, a prevalent tumor suppressor protein (and the focus of several other early-stage biotechs), thereby allowing the tumor to grow unchecked.
GCN2, meanwhile, is a myeloid-derived suppressor cell target that works downstream of IDO and arginase. GCN2 inhibition has the potential for superior efficacy, as it can reverse immune suppression caused by depletion of both tryptophan and arginine.
FLX475 hits the clinic on the strength of preclinical data presented by FLX earlier this year. Those results showed that FLX’s CCR4 antagonists block the migration of regulatory T cells—in the tumor but not in peripheral tissues—and aid the expansion of activated effector T cells. Adding the CCR4 antagonist to anti-PD-L1 and anti-CD137 antibodies dialed up the tumor-killing effects of these drugs.
FLX lists the CCR4 antagonist program as the most advanced in its pipeline, despite having started life with a FLT3 and CDK4/6 inhibitor that was already in phase 1. Flexus moved that drug, FLX925, into the clinic just before being bought by Bristol-Myers. FLX925 has since disappeared from FLX’s pipeline, although a phase 1/1b dose-escalation trial is listed as ongoing on ClinicalTrials.gov.
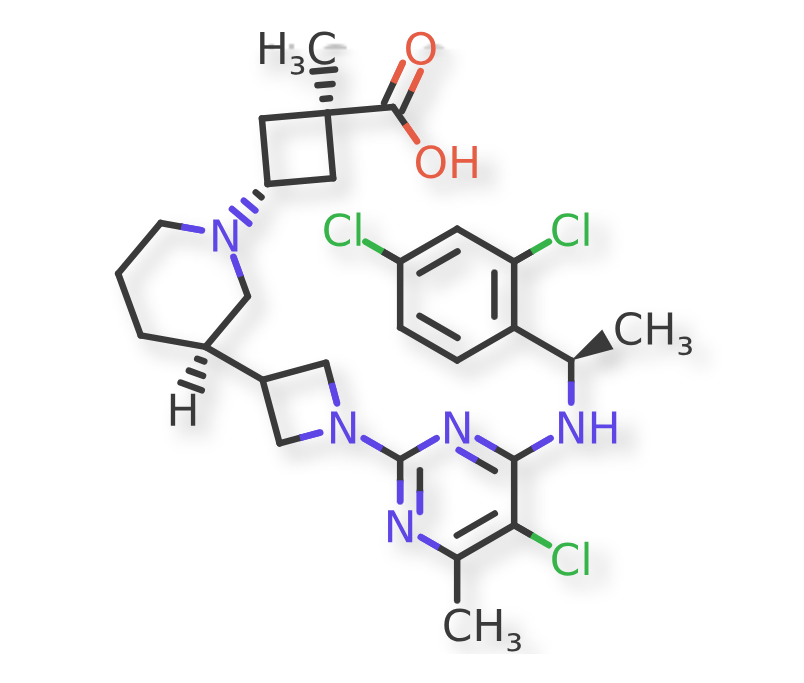
2년 후 FLX Bio는 Immuno-Oncology에 주력하던 Business Model을 Allergy와 Inflammator Diseases로 확대하면서 사명을 RAPT Therapeutics로 바꿉니다. 그리고 오늘 얘기할 Zelnecirnon (RPT193)의 임상시험 계획을 발표했습니다.
FLX Bio keeps it capital in RAPT Therapeutics rebrand – Fierce Biotech 5/24/2019
Now, the biotech is renaming itself RAPT Therapeutics to reflect its expansion into allergy and inflammatory diseases.
“Since our founding, we have internally discovered and advanced two unique drug candidates that target CCR4, with FLX475 in development for the treatment of multiple cancers and RPT193 expected to enter clinical studies in the second half of 2019,” Wong said.
Unlike FLX475, which focuses on Tregs, the inflammation candidate RPT193 takes aim at helper T cells. Tregs suppress other immune cells to shut down the immune response—for example, to prevent autoimmune disease—while helper T cells activate other immune cells. RPT193 is designed to block the movement of type 2 helper T cells into inflamed tissues and reduce inflammation in diseases such as asthma, rhinosinusitis and atopic dermatitis. RAPT plans to file an IND for the drug by the end of the year.
사명 변경을 한 지 한달 후 $37 Million Series C Extension을 했고요.
4개월만에 IPO를 했습니다.
Rapt Therapeutics revives IPO, files to raise $75M – Fierce Biotech 10/23/2019
2019년 12월에는 한국의 한미약품이 Tivumecirnon (FLX475) 의 한국 및 중국판권을 $10 Million upfront 포함 $118 Million 규모의 계약을 맺었습니다.
Rapt edges into Asia with Hanmi I-O deal worth up to $118M – Fierce Biotech 12/3/2019
IPO 후 4개월만에 다시 $75 Million 유상증자를 했습니다.
RAPT Therapeutics Announces Pricing of $75 Million Public Offering – CNN Business 2/7/2020
다시 1년 후에 $144 Million 유상증자를 했습니다.
다시 1년반만에 $75 Million 유상증자를 했습니다.
RAPT Therapeutics $75 million follow-on offering – Davis Polk 11/22/2022
유상증자로 충분한 자금확보를 하고 Zelnecirnon (RPT193)과 Tivumecirnon (FLX475) 의 임상이 잘 진행되던 중 Zelnecirnon의 임상시험을 받던 아토피 피부염 환자의 간기능이 나빠지면서 임상시험이 중단되었습니다. Tivumecirnon (FLX475)의 임상시험은 계속 진행됩니다.
Rapt Therapeutics is reeling from an “unfortunate and unexpected” clinical hold after a patient in a phase 2 immunology trial experienced liver failure that may be related to the study drug.
The serious adverse event occurred in a phase 2b atopic dermatitis trial. The cause remains unknown but Rapt said the event may be “potentially related to zelnecirnon.”
The FDA notified Rapt of the clinical hold on both the phase 2b atopic dermatitis trial and a phase 2a study in asthma.
Rapt noted that roughly 350 patients have been treated with the drug across three trials and no signs of liver toxicity had been reported before now and there has been no evidence of potential liver toxicity in clinical trials. At the end of November, Rapt published phase 1 data of zelnecirnon which found that the drug was “generally well-tolerated” with no serious adverse events and that all reported side effects were mild-to-moderate.
The South San Francisco biotech says that the person had a drug allergy to Dupixent, an autoimmune disease requiring thyroid hormone replacement therapy, contracted COVID-19 during the trial and used “an herbal supplement known to be associated with liver failure.”
That meant little to investors, with the company’s share price nosediving after the markets opened Tuesday, down 64% from $25.97 to $9.25.
With the inflammation program on hold for now, all attention turns to Rapt’s phase 2 oncology med, tivumecirnon, which is being tested alone and in a combo with Merck & Co.’s Keytruda.
Rapt reported $184.8 million in cash on hand at the end of September, enough to last into the middle of 2025.
아토피 피부염 임상2b를 진행하는 중이었는데 QD 50mg, 200mg, 400mg의 3개의 cohort로 진행 중이었고 환자는 268명이 참가한 상태입니다.

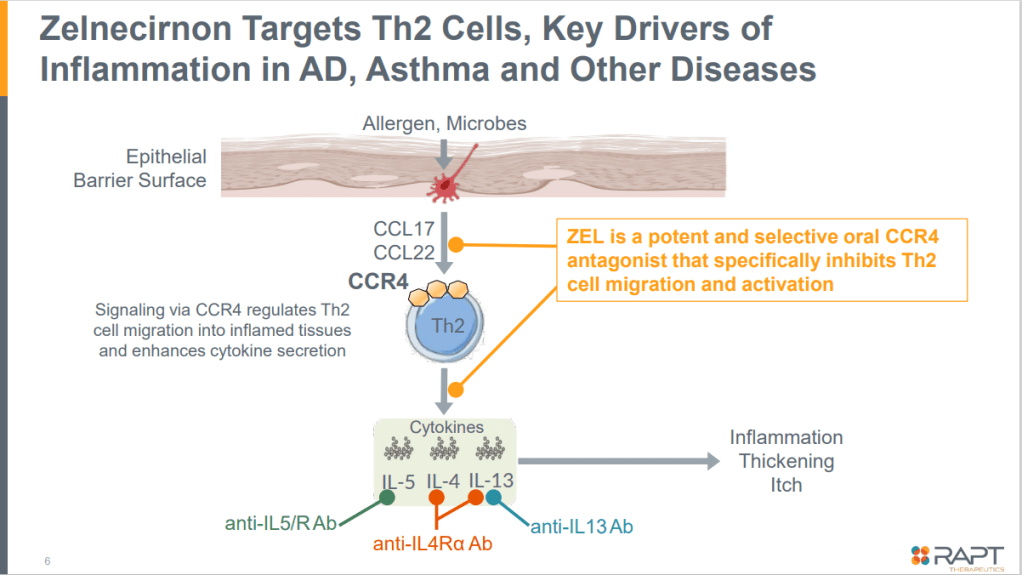
Zelnecirnon (RPT193)의 메카니즘은 아래와 같습니다. helper T cell을 조절합니다.
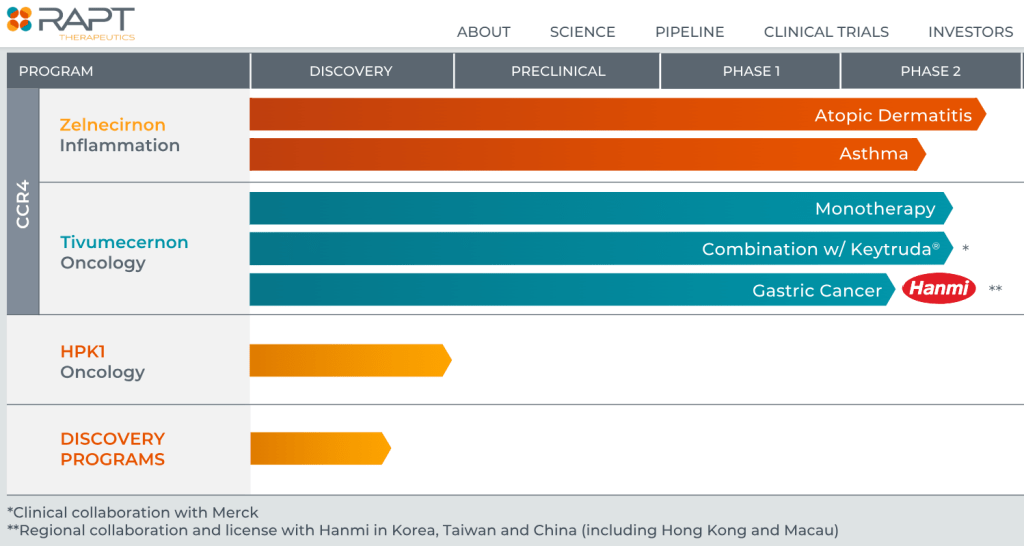
현재 RAPT의 파이프라인은 아래와 같습니다.
2024년 1월에 발표한 회사 Presentation은 아래에 링크합니다.
Clinical Hold를 풀기 위해서 많은 노력을 하리라 생각하는데 당분간 증자는 어려워 보이네요. 건투를 빕니다.
