안녕하세요 보스턴 임박사입니다.
MASH (Metabolic-Associated SteatoHepatitis) 질환은 NASH (Non-Alcoholic SteatoHepatitis)라고 불리던 병의 새로운 이름인데 수십조원대의 시장규모가 예상되는 질환입니다. 다만 Biology에 대한 이해가 여전히 진행 중이기 때문에 여러가지 다른 Mode of Actions에 대한 치료제들이 개발되고 있는 상황입니다. Madrigal의 Resmetirom과 Cymabay의 Seladelpar에 대해서는 글을 의견을 남긴 적이 있습니다.
BIOTECH (10) – Madrigal Pharmaceuticals의 NASH 치료제 MGL-3196 (Resmetirom) 임상 3상 결과

Inventiva Pharma는 2011년에 Abbott Laboratories의 Spin off 회사로 설립이 되었습니다. 이 연구소는 2009년에 Abbott Laboratories가 Solvay Pharma로 부터 인수했는데 인수한지 2년 후 문을 닫기로 결정하게 됩니다. 이에 당시 General Manager였던 Frédéric Cren과 Research Director였던 Pierre Broqua박사가 Abbott과 협상을 통해서 70여명의 직원과 20만개 이상의 파이프라인, 그리고 연구소와 연구팀을 인수하고 Inventiva Pharma를 설립하기로 하게 됩니다. Abbott은 Inventiva를 재정적으로 지원하면서 2개의 프로그램 공동계약을 맺게 됩니다.
Frédéric Cren는 Inventiva의 Cofounder이자 CEO이고 Pierre Broqua 박사는 Cofounder이자 CSO로 창업초기부터 지금까지 이어지고 있습니다.

Abbott Completes Acquisition of Solvay Pharmaceuticals – PR Newswire 2/16/2010
Abbott (NYSE: ABT) today announced that it has completed its EUR 4.5 billion ($6.2 billion) acquisition of Belgium-based Solvay Pharmaceuticals, providing Abbott with a large and complementary portfolio of pharmaceutical products and expanding Abbott’s presence in key global emerging markets.
Co-founded by Frédéric Cren, formerly General Manager of Research at Laboratoires Fournier, and Dr Pierre Broqua, formerly Research Director at Laboratoires Fournier at Daix, Inventiva has a staff of more than 70 experienced people, including around 20 researchers and more than 40 specialized technicians.
In order to achieve its objectives, Inventiva has at its disposal a library of around 200,000 compounds, a 12,000m2 laboratory, state-of-the-art facilities and drug discovery teams grouped into three departments – biology and pharmacology, chemistry and ADME/PK – as well as cross-disciplinary research capabilities (compound management, pre-formulation, toxicology, etc).
In order to promote the start-up and sustainability of Inventiva, Abbott is providing the new company significant financial support. Inventiva is already working on two research programs for Abbott in the field of auto-immune diseases and the treatment of diabetic nephropathy.
Frédéric Cren, Co-Founder and CEO of Inventiva, said: “The launch of Inventiva is the culmination of an ambitious project to create a French leader among biotech firms by drawing on the recognized research capabilities of the Daix site. Inventiva will capitalize on the rapidly growing outsourced pharmaceutical research market to implement significant partnerships with large pharmaceutical groups in its areas of specialization: Parkinson’s disease, oncology, auto-immune diseases and fibrosis. With experienced research teams, already identified research programs and significant development potential, Inventiva has all the necessary assets to become a leading regional and national player in pharmaceutical research.”
Inventiva Pharma는 2017년에 Paris Euronext에 IPO를 합니다.
Inventiva raises 48 mln euros in IPO on Euronext Paris – Euronext – Reuters 2/15/2017
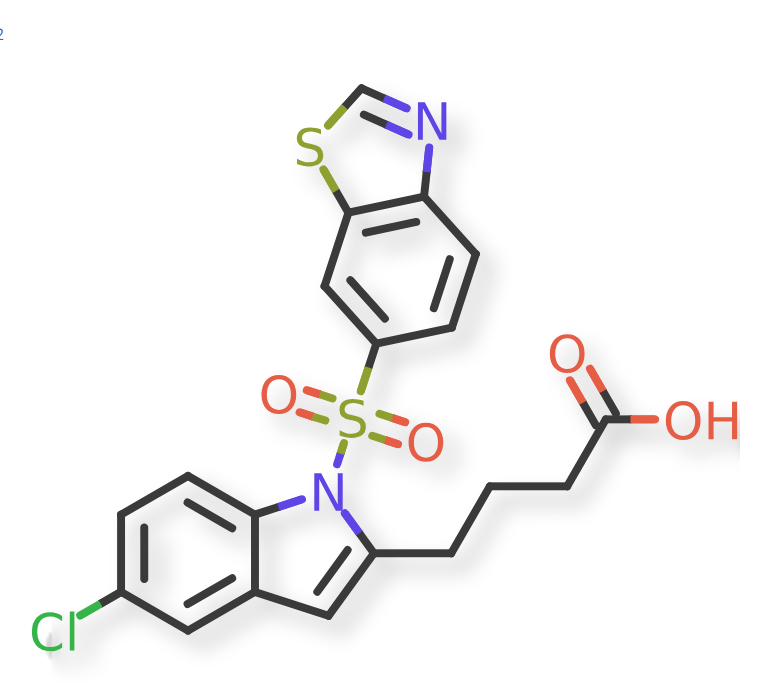
Lanifibranor (IVA337)의 임상2b상 결과를 바탕으로 임상3상을 준비하고 MPS VI or Maroteaux-Lamy syndrome 치료제인 Odiparcil (IVA336) 임상시험을 위해 Nasdaq 상장을 하게 됩니다. 2018년 JMC Paper에 Lanifibranor (IVA337) 개발 스토리를 게재했는데 이 약물이 20만개 이상의 Compound library의 HTS (HIgh-Throughput Screening)결과로 얻어진 Lead compound를 Optimization한 것으로 그 과정을 소개했습니다.
Inventiva aims for $90M Nasdaq IPO after positive phase 2 – Fierce Biotech 6/22/2020
Riding the wave of a phase 2 win, Inventiva is gearing up for its U.S. IPO. The French biotech filed to raise up to $90 million in its Nasdaq debut, which will push its lead program into a phase 3 NASH study and advance a treatment for a rare lysosomal storage disorder.
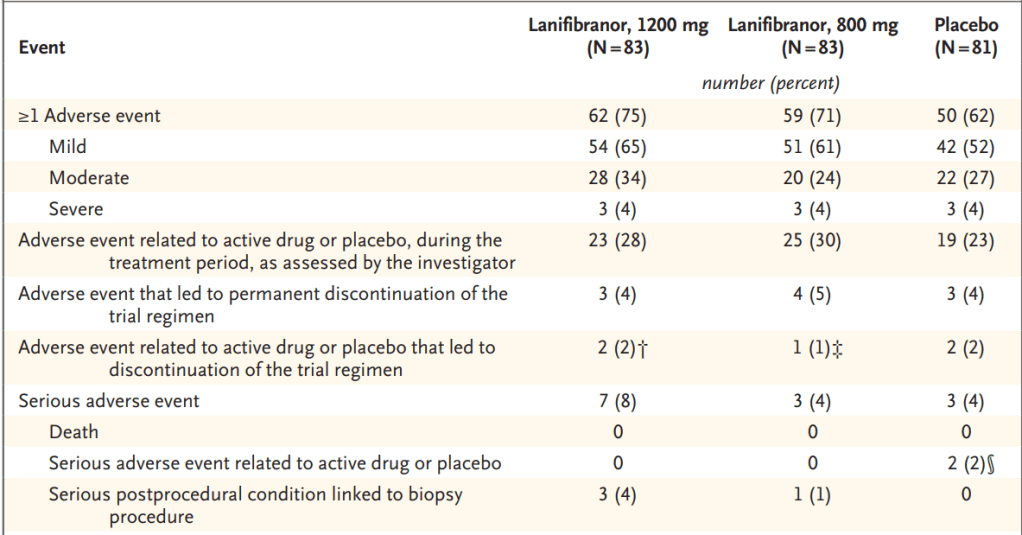
2021년에 Lanifibranor의 임상2b 결과는 NEJM에 발표되었습니다. 800mg과 1200mg에 대해 임상2b를 진행했는데 Primary end point와 Secondary end point는 모두 충족하는 결과로 나왔습니다.

그리고 안전성의 경우에도 800mg의 환자 cohort에서는 Placebo와 유사한 데 1200mg cohort에서는 조금 독성이 있는 것으로 보였습니다.
그런데 임상3상 중에 환자 한명이 Aminotransferase라는 liver enzyme의 레벨이 상승함에 따라 임상이 일시 중단되었다고 발표했습니다.
Inventiva pauses phase 3 MASH study over patient’s raised liver enzymes – Fierce Biotech 2/16/2024
The event marks the first serious adverse reaction reported in any clinical trial of lanifibranor to date, Inventiva pointed out. The trial’s data monitoring committee has reviewed the adverse reaction report “in conjunction with other milder cases of elevation of aminotransferases among trial participants,” the company said.
하지만 Clinical Hold는 아닙니다. DMC는 임상 환자들이 6주마다 간수치 검사를 받고 신규 환자가 autoimmune liver or thyroid disease가 없어야 한다는 조항을 들어 임상진행을 계속할 수 있다고 했기 때문입니다.
The committee recommended that the study can continue if patients receive liver monitoring every six weeks and if new patients diagnosed with or with a predisposition to autoimmune liver or thyroid disease are excluded from the trial. As a result, the biotech has moved to temporarily suspend screening and randomizing any new patients into the study while the criteria are put in place.
CEO인 Frédéric Cren은 임상시험이 1달 반 정도 지나면 다시 시작할 수 있다고 말했습니다.
“All our teams are working diligently, and we are confident that recruitment will resume in around four to six weeks’ time,” CEO Frédéric Cren said in the release. Inventiva had been on schedule to complete patient screening in the trial by the end of March but said the pause means the first visit of the final patient enrolled may occur later in the first half of the year.
2023년 1월에 FDA 권고에 따라 NATIV 임상3상 프로토콜의 수정을 한 상태인데 이 수정을 통해서 임상3상의 시간이 크게 단축될 수 있게 되었습니다.
“following a consultation with the U.S. Food and Drug Administration (“FDA”), Inventiva has decided to modify the clinical development plan of lanifibranor for the treatment of NASH. Inventiva’s request for a consultation with the FDA followed a public communication by the FDA suggesting that an alternative approach to seek full approval in patients with NASH could be considered upon submission of positive results of a Phase III trial using a histology surrogate endpoint in patients with NASH and a Phase III clinical outcome trial in patients with NASH and compensated cirrhosis. The Company’s proposed changes to the NATiV3 trial are designed to align with the alternative regulatory approach and are expected to be beneficial to the overall lanifibranor clinical program by
1) reducing the number of biopsies a patient undergoes during the trial from three to two,
2) reducing the trial duration a patient has to consent to from 7 years to 72 weeks,
3) offering all patients in the trial access to a lanifibranor treatment for at least 48 weeks by allowing them to enter into a new active treatment extension study, and
4) potentially expanding the addressable patient population to include patients with NASH and compensated cirrhosis.”
현재 Inventiva Pharma의 ipeline은 아래와 같습니다. 현재 2개의 약물이 임상시험 중이고 Lanifibranor (IVA337)이 임상3상으로 가장 앞선 상태이고, Odiparcil (IVA336)은 임상2상이 진행 중입니다.

곧 Lanifibranor (IVA337)의 임상3상이 재개되고 승인까지 되어서 MASH 환자들에게 좋은 치료제가 제공되면 좋겠다는 생각입니다.
