안녕하세요 보스턴 임박사입니다.
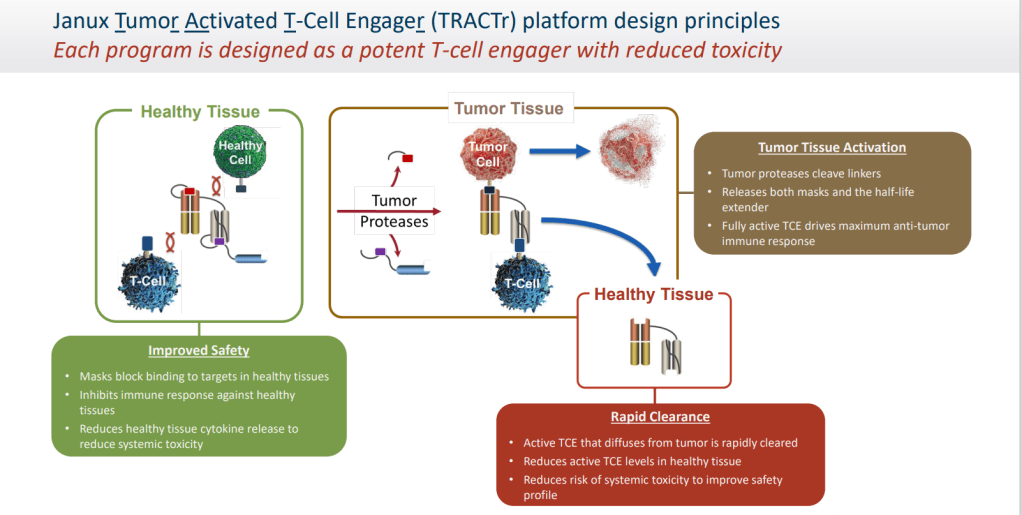
Janux Therapeutics는 Tumor Activated T-Cell Engager (TRACTr) platform을 기반으로 Precision Oncology회사입니다.
Janux Therapeutics는 2017년에 CEO David Campbell 박사가 Founder로서 San Diego에 있는 Early-stage VC Avalon BioVentures의 Incubator인 “COI Pharmaceuticals” 안에서 설립되었습니다. 2018년 5월에 CSO Tommy DiRaimondo 박사가 조인한 후 18개월동안 Stealth-mode로 Tumor Activated T-Cell Engager (TRACTr) platform을 Build-up 한 이후에 Merck와 $1 Billion 규모의 딜을 성사시켰습니다.
Janux pairs up with Merck for $1B-plus T-cell engager deal – Fierce Biotech 12/18/2020
In early preclinical work, its candidates have shown “comparable anti-tumor efficacy relative to standard T cell engagers but lack the associated liabilities related to cytokine release, healthy tissue toxicities, or systemic immune activation.” The proof, however, will need to be shown in clinical trials.
그리고 3개월이 지난 후 $56 Million Series A를 해서 TROP2-TRACTr, PSMA-TRACTr 프로그램 중 하나를 2022년 상반기에 임상시험으로 진입시킨다는 계획이었습니다.
Janux’s proprietary Tumor Activated T Cell Engager (TRACTr) technology is designed to overcome specific limitations of current T cell immuno-oncology therapies. The financing will be used to advance Janux’s preclinical pipeline, including a TROP2-TRACTr and PSMA-TRACTr, with expected advancement of the Company’s first candidate into the clinic in the first half of next year.
Series A $56M 한지 한달후에 $125 Million Series B를 바로 했습니다. 1달만에 EGFR-TRACTr 파이프라인이 새로 들어갔습니다.
The proceeds of the financing will help support the advancement of Janux’s pipeline of next generation T cell engager immunotherapies, including a PSMA-TRACTr, EGFR-TRACTr, and TROP2-TRACTr, into initial proof of concept clinical trials.
Series B를 한지 한달 후에 Nasdaq IPO $100 Million를 발표하고 6월에 계획의 거의 두배인 $194 Million IPO를 했습니다.
Cancer Biotech Janux Therapeutics Files for $100 Million IPO – Biospace 5/21/2021
Janux plans to use the funds raised from the IPO to submit four Investigational New Drug (IND) applications to the U.S. Food and Drug Administration (FDA) in the first half of 2022. The lead program is PSMA-TRACTr for metastatic Castration-Resistant Prostate Cancer (mCRPC). The next two are EGFR-TRACTr for colorectal cancer and head and neck cancer and TROP2-TRACTR for triple-negative breast cancer, urothelial cancer and non-small cell lung cancer. The least advanced program is Costim bispecific, targeting PC-L1xCD28 in solid tumors.
IPO 이후 2023년 중순까지의 스토리는 아래 기사에 잘 정리가 되어 있습니다. PSMA-CD3 TRACTr인 JANX007과 EGFR-CD3 TRACTr인 JANX008의 임상1상이 진행 중이었고 그 결과를 2024년초에 발표할 계획이었습니다.
Janux is building two lines of products using this technology.
The tumor-activated T-cell engager (TRACTr) platform produces T-cell engagers with a tumor antigen-binding domain and a CD3 T-cell binding domain.
In contrast, the tumor-activated immunomodulator (TRACIr) platform produces bispecifics with a tumor antigen-binding domain and a costimulatory CD28 binding domain.
Based on these preclinical insights, the company is conducting a Phase Ia trial in which metastatic castration-resistant prostate cancer (mCRPC) patients will receive JANX007 by intravenous infusion once a week in 21-day cycles. Investigators will increase the dose until a maximum-tolerated dose is identified, and then in the expansion stage, patients will receive doses at previously identified tolerable levels. Investigators are monitoring prostate-specific antigen (PSA) levels and PSMA expression in relation to patient response.
Janux’s second clinical stage program is an EGFR/CD3-directed T-cell engager, JANX008. Based on this, the firm began a Phase I trial of the drug in April in patients with advanced or metastatic solid tumors, focusing on colorectal cancer, squamous cell carcinoma of the head and neck, non-small cell lung cancer, and renal cell carcinoma. Campbell said the company chose those four tumor types because EGFR is overexpressed in anywhere from 50 percent to 95 percent of patients.
그리고 최근 발표한 임상1상 결과는 매우 고무적이어서 하루만에 주가가 3배로 뛰었습니다.
Janux shares triple on early cancer immunotherapy data – Biopharmadive 2/27/2024
Yet Janux’s results, while early and from only a small number of people, suggest its drug could have fewer serious safety risks. None of the 23 treated participants with metastatic castration-resistant prostate cancer experienced severe cases of an immune side effect known as cytokine release syndrome, or CRS, for instance.
Efficacy results, meanwhile, showed treatment led to substantial decreases in a prostate cancer marker that’s indicative of benefit. At higher doses Janux tested, all six treated participants had a 30% or higher decrease in this marker, known as prostate-specific antigen. Five had a 50% or higher decline, and one had a greater than 90% decline.
임상1상 자료는 아래의 회사 Presentation에 잘 나와 있습니다.
JANX007, JANX008의 임상시험이 소규모이고 초기단계이지만 Therapeutic Window를 크게 넓힐 수 있다는 점에서 많은 기대가 됩니다.
