Picture source from Monash University
안녕하세요 보스턴 임박사입니다.
PureTech Health는 영국계 바이오텍으로서 흥미롭게도 회사 내에서 New Platform Startups Incubating을 하고 투자도 할 뿐만 아니라 기술을 사거나 팔기도 합니다. 이 회사의 CEO인 Daphne Zohar와 MIT의 Bob Langer교수가 2005년에 co-founder인 회사입니다. CEO Daphne Zohar의 창업스토리가 재미있습니다.
PureTech Health의 2024년 Corporate Presentation은 아래에 있습니다.
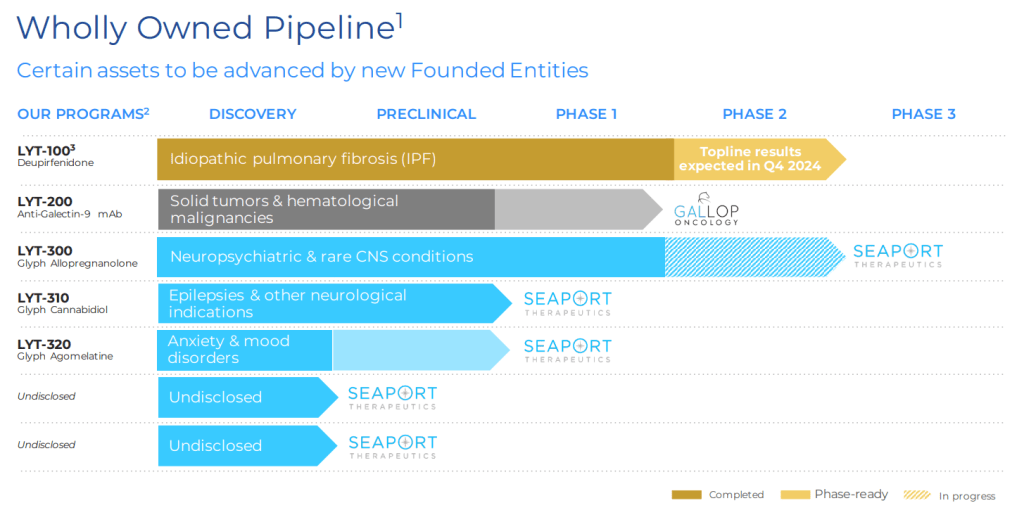
현재 5개의 파이프라인이 있는데 이 중 3개가 Glyph라는 Platform으로 이루어져 있습니다.
Glyph platform 기술은 2016년에 Angewandte Chemie IEE에 최초로 보고하게 됩니다.

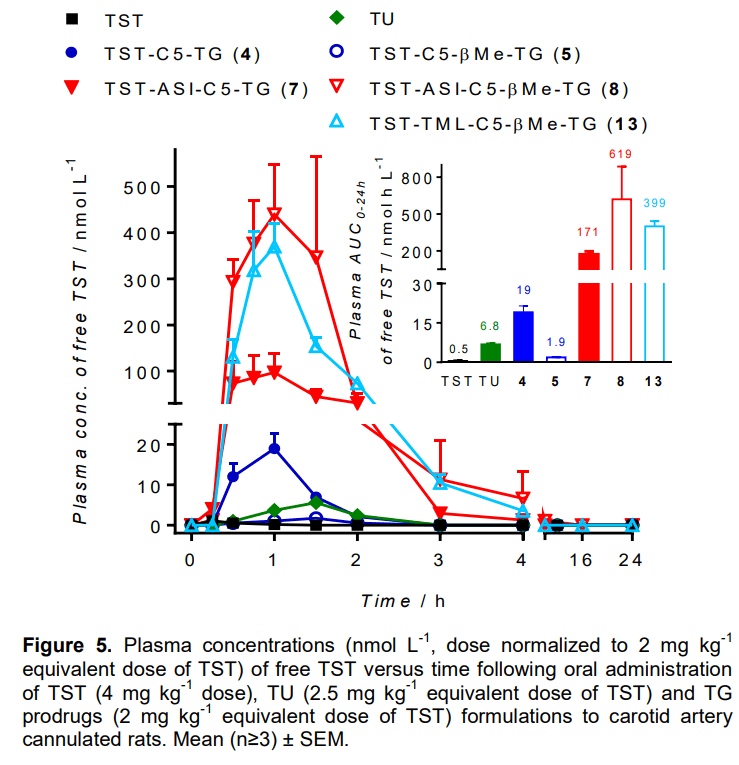
2017년에 PureTech Health는 Australia의 Monash University Chris Porter교수로 부터 Lymphatic delivery prodrug system을 사게 됩니다.
PureTech Health plc (“PureTech Health” or the “company”, LSE: PRTC), an advanced, clinical-stage biopharmaceutical company, today announced an exclusive licensing agreement with Monash University for a lymphatic targeting platform (the Glyph technology) based on the pioneering research of Christopher Porter, Ph.D., director of the Monash Institute of Pharmaceutical Sciences (MIPS) at Monash University in Australia. The Glyph technology is aimed at harnessing the biology of the lymphatic system to develop novel therapeutics, including those that selectively target certain lymph nodes. This program further builds on PureTech’s leadership in identifying novel approaches to address dysfunctions of the brain, immune, and GI systems.
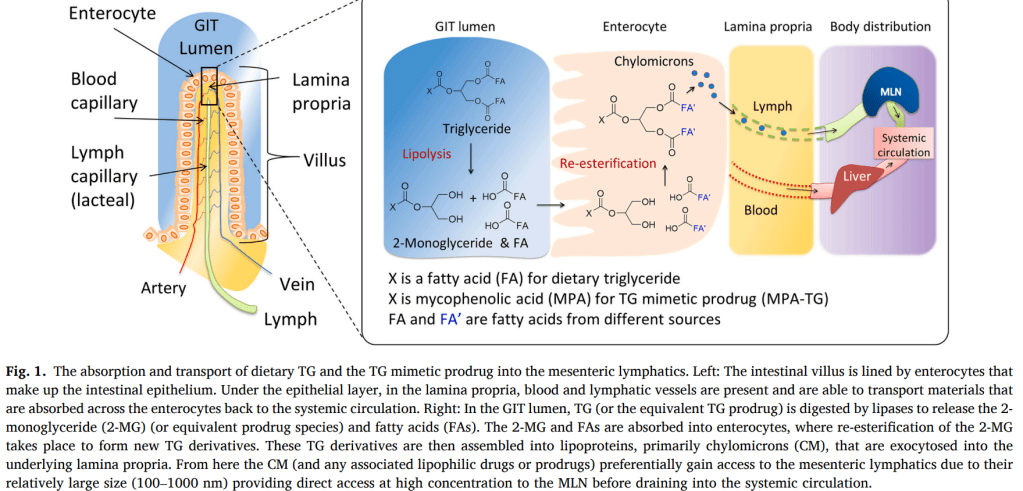
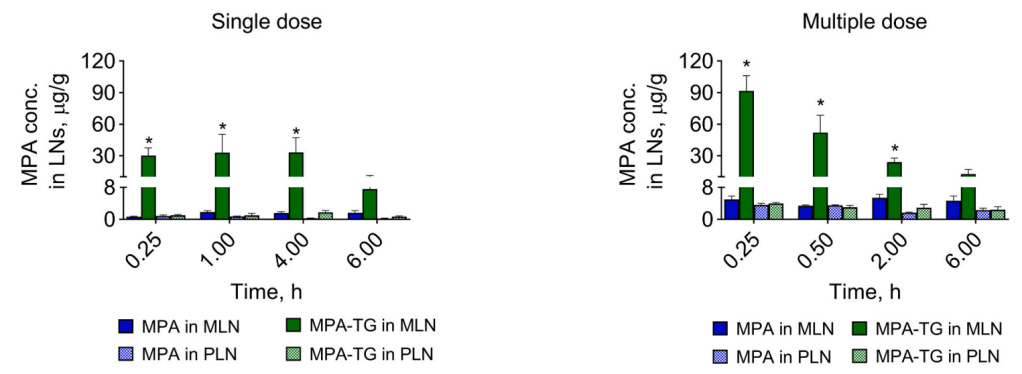
The Glyph technology is designed to harness the biology of the lymphatic system and the endogenous trafficking of compounds through this network to develop novel drugs that bypass first-pass metabolism, improve oral bioavailability, and significantly lower the risk of liver toxicity. In particular, the mesenteric lymph nodes, proximal to the gut, are exposed to a host of microbiome-related species and serve an integral role in immune education and control. Targeting the lymphatic pathway potentially enables rational design of therapeutics to modulate the immune system, representing an innovative approach to treating a broad range of serious immunological disorders, such as cancer and autoimmune diseases. The Glyph technology will be developed by PureTech Health through its subsidiary, Glyph Biosciences, in collaboration with Dr. Porter’s laboratory.
“Through our work at Monash University, we have designed chemistries that potentially enable drugs to be preferentially and effectively transported through the endogenous pathways of lipid transport via the intestinal lymphatics in a controlled manner,” said Dr. Porter. “Our technology has been shown in preclinical experiments to achieve significant oral bioavailability of compounds through the avoidance of first-pass metabolism, and has the potential to significantly mitigate liver toxicity and to alter systemic drug disposition. I am excited to be working with PureTech Health to rapidly advance this potentially disruptive technology platform toward the development of novel therapeutics.”
“This new program builds on PureTech’s unique expertise and approach to utilize novel biology, such as the lymphatic distribution network, to treat serious diseases,” said David Steinberg, chief innovation officer and a cofounder of PureTech Health. “We look forward to a great partnership with Dr. Porter and building on his work at Monash University to drive advancements in immunomodulation.”
2년 후에 Glyph Platform 기술은 Boehringer Ingelheim에 Sub-license 하게 됩니다.
Under terms of the agreement, PureTech Health will receive up to $26 million, including upfront payments, research support, and preclinical milestones, and is eligible to receive more than $200 million in development and sales milestones, in addition to royalties on product sales. The collaboration will initially focus on applying PureTech’s lymphatic targeting technology to an immuno-oncology product candidate designated by Boehringer Ingelheim.
More specifically, the therapeutic candidates are directed to the mesenteric lymph nodes, which program as many as 70 percent of circulating adaptive immune cells. PureTech’s lymphatic targeting approach, which is based on the research of Chris Porter, PhD, Director of the Monash Institute of Pharmaceutical Sciences (MIPS) at Monash University, can potentially be applied to therapeutic molecules across a range of physiochemical properties and holds promise for the development of novel therapeutics for gastrointestinal, central nervous system, and autoimmune diseases as well as cancer.
The research collaboration with Boehringer Ingelheim will focus first on using this approach to administer an immuno-oncology candidate for gastrointestinal (GI) cancers directly to the gut lymphatics.

PureTech Health는 LYT-300을 FXTAS라는 신규뇌질환 치료제 가능성을 위해 DoD grant $11.4 Million을 받기도 했습니다. 이것은 DoD로 부터 받는 4번째 Grant로서 PureTech Health는 그동안 wholly-owned program 개발을 위해서 grant를 받는 형태로 노력해 왔습니다.
The funds will support a Phase 2 trial of LYT-300 in collaboration with the University of California, Davis (UC Davis). An exploratory, open-label trial of six men with FXTAS, evaluated IV-administration of allopregnanolone across multiple neuropsychological and emotional tests. In addition to being well-tolerated, allopregnanolone showed signals of pharmacologic benefit across multiple neurological endpoints, including the Behavioral Dyscontrol Scale, which measures executive, cognitive and motor function, and demonstrated improvement compared to baseline (p=0.009). IV administration is not feasible in most indications, especially for a chronic therapy, and there remains a need for treatments that can address this debilitating condition. PureTech plans to evaluate LYT-300 in a placebo-controlled trial to demonstrate the safety, tolerability and efficacy of the drug in people with FXTAS.
PureTech’s capital efficient strategy includes the pursuit of non-dilutive funding in the form of grants. This is the fourth DoD grant that PureTech has secured on behalf of its Wholly Owned Programs in addition to five grants secured on behalf of its Founded Entities.
2023년 11월에 발표한 LYT-300의 임상결과는 primary end point를 모두 충족했다는 좋은 뉴스였습니다.
PureTech’s acute anxiety treatment trial meets primary endpoint – Clinical Trials Arena 11/15/2023
Puretech Health has reported that its Phase IIa clinical trial of LYT-300 (oral allopregnanolone) to potentially treat acute anxiety met the primary endpoint.
The proof-of-concept, placebo-controlled, randomised trial analysed the salivary cortisol response induced by LYT-300 based on the Trier Social Stress Test (TSST), an established anxiety clinical model.
The trial enrolled 80 healthy subjects who were randomised into a 1:1 ratio to receive either LYT-300 or a placebo.
According to the trial’s topline data, oral LYT-300 offered a statistically significant decline in stress hormone response, as evaluated by salivary cortisol, compared to a placebo, meeting the primary endpoint.
Glyph platform과 LYT-300 임상 개발에 대한 스토리는 아래 Monash University에서 잘 다루었습니다.
LYT-300 was generally well-tolerated across the study, and no treatment-related severe or serious adverse events were observed. The data suggest the potential to dramatically increase practicality and usability of allopregnanolone for those with PPD, along with a broad range of neurological and neuropsychiatric conditions. Additional data will be presented in a scientific forum, and a Phase 1b/2a clinical trial is expected to begin in the first half of 2023.
The LYT-300 trial is the first clinical validation of the Glyph technology in humans.
A second candidate from the Glyph platform, LYT-310 (oral cannabidiol [CBD]), has also been nominated as a clinical candidate and is designed to greatly expand the therapeutic application and potential of cannabidiol (CBD).
Applying the Glyph technology to CBD to form LYT-310 has been shown to increase oral exposure three to fourfold versus unmodified CBD in multiple preclinical models, including large animal and non-human primate. This has the potential to translate into improved safety and reduced side effects. Lymphatic transport has also been confirmed in preclinical models, with up to 30% of LYT-310 entering the lymphatics, compared to 5% for unmodified CBD. These data further support the novel Glyph mechanism of enhancing oral bioavailability. LYT-310 is expected to enter the clinic in Q4 of 2023.