
안녕하세요 보스턴 임박사입니다.
BlueRock Therapeutics는 Memorial Sloan Kettering Cancer Center의 Lorence Studer 박사의 MSK-DA01 (Bemdaneprocel, BRT-DA01)의 임상시험 및 상용화를 위해 Versant Ventures와 Bayer가 공동으로 설립한 회사입니다. Bemdaneprocel story에 대해서는 CSO Stefan Irion 박사가 2023 Till & McCulloch Meetings에서 발표한 것을 정리한 아래의 블로그 글을 보면 알 수 있습니다. 정리하면 2000년대 초까지 Parkinson Disease Cell Therapy를 찾기 위한 exploratory clinical trials를 했는데 그 중 “Fetal Cell Approach”만이 효과가 있었고 Fetal Cell의 생산성에 대한 문제로 Pluripotent Stem Cell을 찾기 시작하고
- 2011년에 Nature에 논문을 내고 2014년에는 G-Force PD라는 컨소시엄을 만들었다.
- “Biphasic Wnt Signaling Activation” 방법으로 Clinical-grade doparminergic neuron을 만들어낼 수 있었다.
- 2021년에 Cell Stem Cell에 Bemdaneprocel (BRT-DA01, MSK-DA01)의 preclinical 결과를 냈는데 (1) PCR로 검출가능한 여러 분화 마커를 찾았고 (2) Rat 실험에서 Functional Cure를 확인했다. – 이 결과 BlueRock Therapeutics가 만들어졌다.
- 2021년에 임상1상을 시작해서 12명에 대해 수술적 방법으로 뇌에 구멍을 내어 Bemdaneprocel 을 이식하고 1년간 immunosuppressant를 복용했다. 1.8-5.4 milion cells를 주입한 경우 고용량에서 효과를 보았다.
- PET를 통해서 뉴런세포가 1년간 생존할 뿐만 아니라 주위 뇌세포로 커가는 것을 볼 수 있었다.
- 2024년 상반기에 임상2상을 할 계획이다.
BlueRock Therapeutics has generated considerable media buzz with their announcement of positive Phase I clinical trial data on bemdaneprocel (BRT-DA01), an investigational, stem cell-derived, dopaminergic therapy for Parkinson’s disease. The trial is the result of decades of research and investments that yielded this exciting step into clinical translation.
Founded in 2016 by Versant Ventures and Bayer LEAPS, with scientific founders Drs. Lorenz Studer (Sloan Kettering Institute) and Gordon Keller (University Health Network), the mission of BlueRock Therapeutics is to discover and develop novel stem cell-derived therapies to improve treatments for patients. With the recent clinical trial, bemdaneprocel is the company’s most advanced product. Providing insights on the bench-to-bedside journey of bemdaneprocel, Dr. Stefan Irion (Chief Scientific Officer, BlueRock Therapeutics) presented an insider’s view on the investigational cell therapy at the 2023 Till & McCulloch Meetings.
As recapped by Irion, cell therapies for Parkinson’s disease began to emerge in clinical trials in the late 1990s and early 2000s. In these early trials, several different dopamine-expressing cell types were explored, including retinal pigmented epithelial cells, autologous carotid body cells, adrenal medullary cells and fetal cells. With the exception of the latter, trials involving these various cell types were largely unsuccessful.
The fetal cell approach was marked by several limitations, including a limited supply of cells and substantial heterogeneity of the cell population that may have compromised efficacy. As such, research turned to pluripotent stem cells as a potential source of renewable cells that could provide a consistent, scalable, and more refined source for generating dopamine-expressing neurons. In parallel, a global consortium called G-Force PD was formed in 2014 by leaders in the field, including Studer, the eventual scientific cofounder of BlueRock Therapeutics. The purpose of the consortium was to gather consensus and inform the intelligent design of a next wave of clinical trials involving stem cell-derived neurons for Parkinson’s disease treatment.
In 2011, the lab published a ground-breaking Nature paper detailing the controlled generation of midbrain dopaminergic neurons from human embryonic stem cells. This study provided an important foundation and led to millions in grant funding to develop the cell therapy for clinical trials. To do so, the team needed to adapt the protocol to make it more efficient and scalable, landing on a biphasic Wnt signaling pathway activation strategy for producing clinical-grade dopaminergic neurons that they reported a decade after the seminal Nature paper.
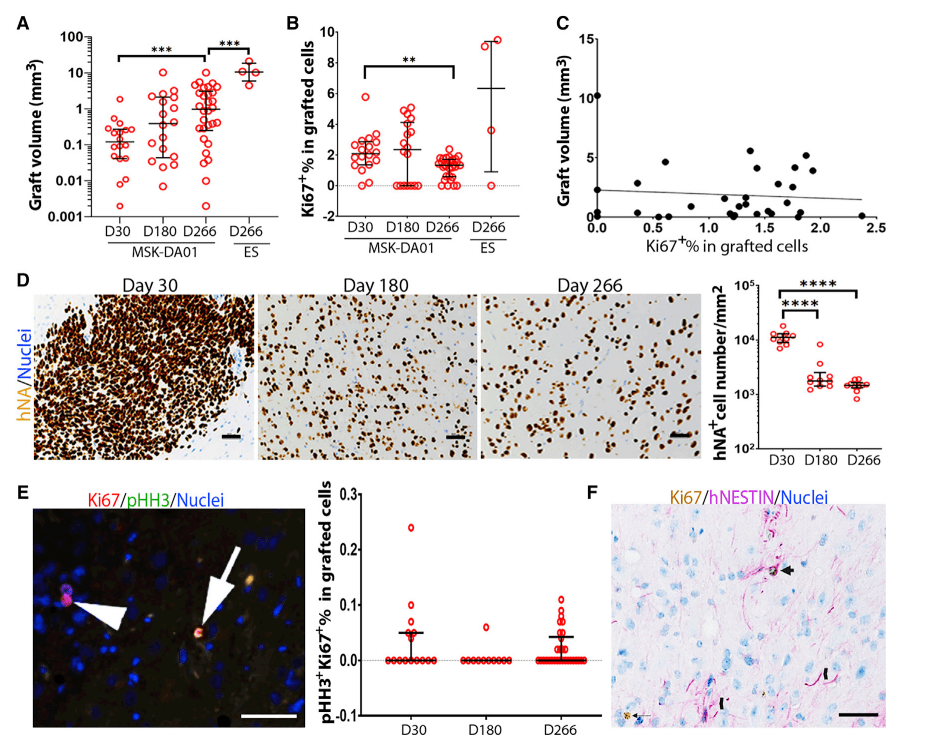
Using the new differentiation protocol, the Studer lab performed extensive preclinical testing of bemdaneprocel, publishing their findings in a Cell Stem Cell paper in 2021. These studies included the identification of a panel of differentiation markers measured by PCR that could be used to validate the phenotype of the cell product and ensure stability after cryopreservation and passing through a delivery device. Using a mouse model, the group performed biodistribution, toxicity, and tumourigenicity studies to demonstrate safety of the treatment.
Studer group showed that rats implanted with bemdaneprocel displayed correction of the rotational bias compared to control rats, providing important evidence for their functional recovery. With these promising preclinical safety and efficacy data, BlueRock Therapeutics initiated plans for clinical trials in humans. Drawing upon previous work in fetal cell clinical trials as well as the rodent studies, the investigators determined estimates for the dose of bemdaneprocel that would be needed for treating human patients with Parkinson’s disease.
Using the body of preclinical data, BlueRock Therapeutics received approvals from Health Canada and the U.S. Food and Drug Administration to conduct a phase I clinical trial. Beginning in 2021, the trial sites included Toronto Western Hospital, University of California Irvine, Weill Cornell Medical College, and Memorial Sloan Kettering Cancer Center. The open-label trial enrolled 12 patients, with the first five patients receiving a low dose of bemdaneprocel (1.8 million cells), and the subsequent patients treated with the high dose (5.4 million cells). A novel surgical technique and device were also developed to precisely implant the cells into the brain via a single burr hole through the skull. The patients will be followed for two years, and Irion presented the one-year interim results that have now been collected for the full cohort. They received immunosuppression for one year after treatment to allow time for the blood-brain barrier to heal from the initial surgery so that it could protect the implanted cells from immune recognition thereafter.
While efficacy data need to be interpreted with caution given that the study is a small open-label trial with risk of placebo effects, clinical scoring systems suggest that patients have improved, particularly those within the high-dose cohort. In addition, positron emission tomography (PET) imaging was performed in patients to measure uptake of a synthetic dopamine analogue and radiotracer to visualize regions of the brain containing dopaminergic neurons. These measurements revealed that the implanted cells survived and persisted at one year follow-up, with engraftment in the expected brain region. In light of this encouraging data, BlueRock Therapeutics has announced plans to begin enrolling patients for a Phase II clinical trial within the first half of 2024. The study will provide new insights on the efficacy of the bemdaneprocel for patients with Parkinson’s disease.
Bemdaneprocel (BRT-DA01, MSK-DA01)의 전임상 연구결과는 Cell Stem Cell 2021년에 보고했습니다.

Bayer, Versant join forces on $225M Series A for stem cell upstart – Fierce Biotech 12/16/2016
The startup, which is being hailed by the pair as a “next-generation regenerative medicine company” plans to develop best-in-class induced pluripotent stem cell (iPSC) therapies. Its initial focus will be on CNS and CV disease areas.
On the R&D side, it will have ops in Toronto, New York and Boston, with its initial program focused on regenerating heart muscle in patients who have had a heart attack. The program is being worked on in a collab pact with the Toronto-based McEwen Centre for Regenerative Medicine and University Health Network with Dr. Gordon Keller, a leader in stem cell biology, and a scientific co-founder of BlueRock.
BlueRock disclosed that one patient temporarily developed seizures a day after the surgery, although the cells themselves did not cause any serious side effects. That 70-year-old man recovered after receiving medication and hasn’t had any seizures since, Ettenberg said.
One year after the surgery, the seven patients who got the high dose of the therapy had a 13-point reduction on a scale that doctors use to measure the motor symptoms of Parkinson’s, and the five patients who got a low dose had a 7.6-point reduction. A 5-point reduction has historically been considered clinically significant. Patients receiving the high dose also cut their time spent with uncontrollable movements — so-called “off time” — each day by about 2 hours.
“We saw an almost around 50% decrease in their off time, which means they’re gaining control of those movement disorders,” Ettenberg said. “If we were writing the script two years ago, this is what we would be hoping for. We’ve gotten everything we need to move this to Phase II.”
Rather than injecting stem cells directly into a patient, the company grows its stem cells into dopaminergic neuronal precursor cells in the lab before a surgeon implants them into each side of a patient’s skull. The goal is to help rebuild the network of dopamine-producing cells in a brain region called the putamen, which is vital for controlling movement but is damaged in Parkinson’s disease. The patients in the study were diagnosed with the condition nine years earlier, on average. And although they were still getting some benefit from levodopa — essentially a dopamine replacement that is the standard of care — all started to lose the full benefit of the treatment over time.
Most of the mild and moderate adverse events were related to immunosuppressant drugs that patients took for one year after the surgery, Ettenberg said. There were no serious adverse events related to the cell therapy, he added, and the seizures one man developed were “somewhat expected for us, and for the field, given that you’re passing a cannula into each side of the brain.”
Since no one got a placebo in the study and there was no control arm, Ettenberg was careful to note that this study was not a true test of the therapy’s effectiveness. “It’s not powered to speak to the clinical benefit here. But of course, we’re all interested in that. And the patients are interested in that. And we need to speak to it,” he said.
The randomized placebo-controlled Phase II trial will seek to more strongly answer questions about the treatment’s efficacy and durability. “We’re still in discussion with the FDA of exactly what that trial design would look like,” Ettenberg said.
These iPSCs partly resemble embryonic stem cells that can be turned into any other cell but with fewer ethical quandaries, and therapies made from iPSCs are only beginning to move from the lab bench and into the clinic. BlueRock’s treatment was made from a cell line that originally came from embryos — fertilized eggs that were never implanted in a patient.
Ettenberg said academic work on those cells started in 2009, three years after iPSCs were made in a petri dish for the first time. Using embryonic stem cells “was the fastest” way forward, he said, adding that there was no fetus involved in making the stem cells, and that the company can perpetually generate new cells from the ones they already have.
“All of the source for this material is already in the freezer. And we will never go back and create another source for that,” he said. “It’s what attracted me to this field in general. You’re making a reproducible, reliable, indefinite source of this material, which can become more drug-like in its characteristics.”
The Parkinson’s treatment is BlueRock’s most advanced program, though the rest of the company’s earlier-stage work focuses on therapies made from iPSCs. BlueRock is getting ready for a Phase I clinical trial with Opsis Therapeutics, a subsidiary of Fujifilm, to make retinal cells for inherited vision diseases. Another program focused on making cardiomyocytes — heart muscle cells — is close behind.
Earlier this month, BlueRock laid off about 50 employees, and Ettenberg said the cuts were expected to help tighten the company’s focus on the next phase of its Parkinson’s program and upcoming retinal disease treatment.
Series A 한 지 3년 후에 Bayer는 $1 Billion Valuation으로 BlueRock Therapeutics를 인수하였습니다.
Following a 2016 joint venture with Versant Ventures to establish BlueRock Therapeutics, Bayer will acquire the remaining stake for approximately USD 240 million in cash to be paid upfront at closing and an additional USD 360 million payable upon achievement of pre-defined development milestones. With Bayer currently holding 40.8 percent stake, the investment corresponds to a total company value of BlueRock Therapeutics of approximately USD 1 billion. The closing of the transaction is expected during the third quarter of 2019.
2023년 중순에는 Bayer 실적 악화로 12%의 인력감축이 있었습니다.
The Bayer-bought cell therapy biotech is taking a pipeline of nine assets and focusing on four, according to a statement from a BlueRock spokesperson. The priority is the company’s cell therapy to treat Parkinson’s that’s gearing up for a phase 2 trial set to begin in the first half of 2024. Ophthalmology asset OpCT-001 will soon join it in human trials, with a formal ask to regulators expected sometime in the next year. BlueRock will also continue work on a second Parkinson’s cell therapy and a stem-cell-derived treatment for heart failure.
The 12% reduction represents layoffs to about 50 employees, the spokesperson added. The cuts will be across sites in Cambridge, New York and Toronto.
Bayer opens California plant to make cell therapies – Biopharmadive 10/10/2023
Bayer has been investing hundreds of millions of dollars into its Berkeley location, recently launching a Cell Culture Technology Center and cell therapy labs.
지금의 속도로 간다면 임상2상을 마치는 시점에서 FDA Accelerated Approval을 시도할 것 같습니다. 최초의 Parkinson Disease iPSC-derived neurologic Stem Cell Therapy가 되어 환자들에게 희망을 줄지 기대됩니다.

One thought on “BIOTECH (99) BlueRock Therapeutics: Bemdaneprocel (BRT-DA01, MSK-DA01) – Autologous Stem Cell Therapy for Parkinson’s Disease”