
안녕하세요 보스턴 임박사입니다.
GenEdit은 Hydrophilic Polymer Nanoparticle을 Gene Delivery System으로 개발하는 회사로서 UC Berkeley의 박효민 박사와 이근우 박사 그리고 Niren Murphy교수가 공동 창업한 회사입니다.
Under the terms of the agreement, GenEdit has granted Editas Medicine an exclusive worldwide license, with rights to sublicense, to GenEdit’s Cpf1-based technologies. In return for these rights, GenEdit will receive undisclosed upfront and development milestone payments, including royalties on net sales of products incorporating the licensed intellectual property. In addition, GenEdit and Editas Medicine will collaborate on evaluating delivery of Cpf1-based technologies with GenEdit’s nanoparticle platform. Editas Medicine will provide research funding and have an option to continue development after the initial collaboration period.
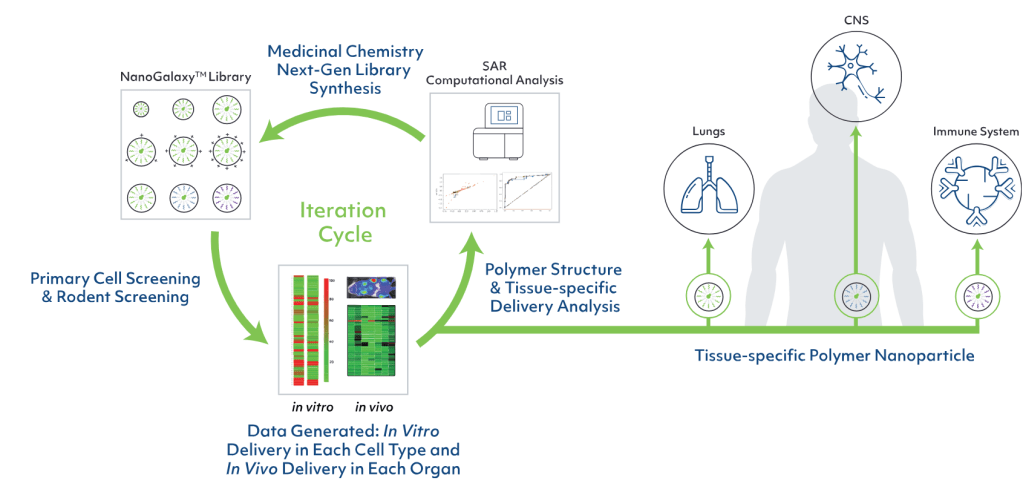
The company screens the library to identify initial hits and then uses computational analysis and medicinal chemistry for iterative lead optimization. The company has used this platform to identify multiple candidate polymers for efficient and specific delivery of gene editing to a range of tissues.
“Compared to viral vectors and lipid-based nanoparticles, our approach has the potential for better targeting, more cargo, and lower manufacturing cost,” said Timothy Fong, Ph.D., chief scientific officer of GenEdit. “In particular, our approach has the potential to enable in vivo gene editing of multiple tissues with CRISPR and expand the potential of gene therapies to treat more diverse sets of diseases.”
It was Christmas Eve in 2015. The seasonal festive vibe was blocked out from the science lab in UC Berkley, California, where bioengineering doctoral candidate Lee Gun-woo, then 27, was immersed in his research. Lee’s heart quickened as he watched the results of his experiment unravel before his eyes. A polymer nanoparticle traveled straight to his intended target genome in a test rat and changed its DNA. A jolt ran down his spine. “This could make CRISPR DNA scissors work in the human body. It could be a game changer in delivering drugs to tackle genetically caused illnesses,” he thought. Lee dialed up his friend and fellow scientist Park Hyo-min. “Hyeong, let’s start a company,” Lee said.
The story of Lee Gun-woo, 33, and Park Hyo-min, 41, is truly an American Dream come true. The duo co-founded the gene therapy company GenEdit in May 2016 with Lee as the chief executive officer leading research and Park as the chief technology officer responsible for validation of development. What GenEdit does is step up the game for the groundbreaking DNA scissors technology called CRISPR. The technology snips parts of the human DNA to remove or insert new genetic material. The Nobel Prize for Chemistry in 2020 went to scientists Emmanuelle Charpentier and Jennifer Doudna for their contribution to its discovery. Lee and Park are not strangers to the leading CRISPR researcher Doudna, who is a professor at their alma mater UC Berkley.
Doudna’s name is listed as a contributor to Lee and Park’s co-authored article published in Nature in 2017, titled “Nanoparticle delivery of Cas9 ribonucleoprotein and donor DNA in vivo induces homology-directed DNA repair.” Lee, Park and scientist Michael Convoy have equal stakes in the piece as main authors, according to the science journal.

Born out of UC Berkley’s laboratory, GenEdit’s polymer nanoparticle delivery technology can make the Cas9 travel to the desired location in human body. This innovative concept has attracted global angel investors such as the likes of Sequoia Capital, Bow Capital, Data Collective Bio and SK Holdings. The Series Seed financing round raised a total of $8.5 million, led by DCVC Bio and SK Holdings.
Now in its fifth year, GenEdit aspires for the future where all hereditary illness can be cured with gene therapies. There are countless ailments that are passed down the blood line and GenEdit is studying DNA markers for non-viral polymers that will match them. Speaking to The Korea Herald in a video interview, CTO Park Hyo-min said that GenEdit will soon decide on the first target disease within 2021.
“We’re not definitive at this point, but there is a good chance we will narrow down our focus to central nervous system diseases, for which we have been able to amass a volume of promising data,” Park said. “It is not with absolute certainty I say this, but we may be able to deliver a novel drug for a rare CNS disease within the next six to seven years,” said Park, if authorities fast-track their approval for orphan drugs for rare diseases.
Other than CNS indications, the company is also perusing therapies for liver and immune cell diseases.
“The liver has comparatively low hurdle for drug delivery mechanisms,” Park said, “Precisely for that reason there is much competition in the area of liver treatments, but we may still consider to throw our gloves in.” Apart from their main target pipeline, for which GenEdit intends to see through to drug commercialization, the company is open to strategically licensing out other findings, Park said. When asked what motivates these researches, and what does it feel like to be a young, celebrated scientist, CEO Lee Gun-woo — who is eight years younger than co-founder Park — remained modest.
“Through this winding long process of life, I dream of serving the society in any way I can. To be able to serve, I believe it’s imperative to broaden my capabilities,” Lee said.
“I had the opportunity to listen to astronaut Jonny Kim’s webinar. He spoke of Martin Luther King and the life of service, that everyone is capable of greatness through the act of giving,” Lee said. “One of my dreams is to use science to benefit more patients, and I am profoundly grateful that I am on that path.”
The young co-founders of GenEdit had not foreseen that they would be leading a gene therapy company in the US when they first set foot on US soil in 2011. Lee had come straight after his bachelor’s degree in bioengineering at Korea Advanced Institute of Science and Technology in Daejeon, and Park had come after completing a master’s in food science at Korea University in Seoul. Lee had spent most of his life growing up in his home city of Daegu, while Park had lived in Seoul.
“I would like to tell scientists in Korea that they must create reasons to come out to the US. Korea has great science, but one can’t deny that it’s here in the US where all the breakthrough innovations happen,” said Park.
“If in Korea, every academic novelty would have to be indirectly studied. Here, everything becomes a raw, immediate experience. We get to be in the heart of the research leading scientific progress, shoulder-to-shoulder with Nobel laureates,” Park said.
What binds Lee and Park together as partners is the deep trust and camaraderie that has built up in the decade they have known each other. The five years in school and five years in business have united them as near-family and made them an inseparable team.
As of February, GenEdit had 17 full-time employees, of whom 14 were researchers. By the end of the year, GenEdit plans to boost the headcount to 27 full-time workers. Lee and Park said that they have built a culture where it is OK to make mistakes and keep matters transparent. Anyone who wants to try some cool science and do fun researches is welcome to join this science-focused team, they said.
GenEdit believes its polymer nanoparticles have the potential to avert the safety problems of some gene therapy delivery methods, and the five-year-old biotech now has $26 million to work on proving it, thanks to an investor group that includes the likes of Eli Lilly.
Derived out of a lab at the University of California, Berkeley, GenEdit also shared new in vivo data on its polymer nanoparticles showing tissue-selective delivery and the ability to maintain functional activity after repeat dosing, the biotech said Thursday.
The goal is to overcome the safety problems that can come with adeno-associated virus, or AAV, a viral vector used to deliver gene therapies. Problems with that mode of delivery triggered an FDA advisory committee meeting earlier this month, where experts discussed the future of preclinical research and how to deliver the treatments safely.
“The data presented today indicates we can overcome the historic challenges in the field of gene therapy and establishes the feasibility of using GenEdit’s polymer nanoparticles to deliver genetic medicines to a variety of tissues, including the [central nervous system], with the potential for delivering a therapeutic effect,” said Kunwoo Lee, Ph.D., CEO and cofounder, in a statement.
GenEdit’s platform houses thousands of polymers that are chemically distinct and can deliver DNA, RNA or CRISPR ribonucleoprotein, depending on whether the goal is to add, delete, edit or silence a gene.
The financing and data come a day after GenEdit named Romuald Corbau, Ph.D., as chief scientific officer and Aaron Mishel as chief financial officer. Corbau previously led the research team working on AAV-based gene therapies at Freeline, and before that he was translational lead at Spark Therapeutics. Mishel joins from Magnetic Insight, where he held the same post.
Lilly joins the South San Francisco biotech’s roster of backers, which includes DCVC Bio, Sequoia Capital, Korea Investment Partners and nearly a dozen other investors.
GenEdit has been working off an $8.5 million seed financing from December 2018.
Initial in vivo results from the research collaboration between GenEdit and Sarepta have demonstrated the potential of GenEdit’s polymer nanoparticles to deliver therapeutic cargo to specific muscle tissue after systemic administration to allow for targeted, non-viral systemic delivery of genetic medicines. The research collaboration and option agreement commenced in December 2020.
“We’ve been impressed with the diversity of GenEdit’s NanoGalaxy platform and its screening and selection process, which has generated a number of distinct polymers that deliver to muscle,” said Doug Ingram, president and chief executive officer, Sarepta Therapeutics. “Sarepta is committed to the development of therapies for rare neuromuscular diseases, and we look forward to continuing to work with the team at GenEdit to advance effective gene editing-based treatments for these patients.”
GenEdit has demonstrated in preclinical studies that its NanoGalaxy platform can selectively deliver to different tissues a variety of functional genetic medicine cargos, including CRISPR-Cas9 ribonucleoprotein, for targeted in vivo gene editing.
In addition to research payments, under the terms of the collaboration and option agreement, GenEdit may receive up to $57 million in near-term payments and is also eligible for significant future development, regulatory and commercial milestones and tiered royalties ranging from upper-single to low-double digits on future product sales.
GenEdit Is a Phase I Winner of the NIH TARGETED Challenge. – Press Release 12/13/2023
The Targeted Genome Editor Delivery (TARGETED) Challenge is a $6,000,000 challenge to improve the current state of in vivo delivery technologies for genome editors in two Target Areas: 1) Programmable Delivery System for Gene Editing and 2) Crossing the Blood-Brain Barrier. The Challenge is a three-phase competition. In Phase 1, participants were asked to submit a proposal describing their proposed solution and how it addresses the requirements for one of the Target Areas.
Addressing the American Society of Gene and Cell Therapy Annual Meeting in 2016, four years before being awarded a share of the Nobel Prize in Chemistry for pioneering research in CRISPR, Jennifer Doudna, PhD, began by sharing what has become an oft-repeated maxim in gene therapy by summing up the field’s biggest challenges:
“Delivery, delivery, delivery.”
Among those listening intently was Kunwoo Lee, PhD, a Korean postdoc in her University of California, Berkeley lab. That year, Lee joined two other collaborators with Doudna’s lab in launching a company focused on developing genetic medicines with tissue-selective in vivo delivery.
Lee recalled Doudna’s words this week as his company, GenEdit, signed a major multi-year collaboration and license agreement with Genentech, potentially worth up-to-$644 million, to discover and develop novel nanoparticles capable of delivering the Roche subsidiary’s nucleic acid-based treatments for autoimmune disease.
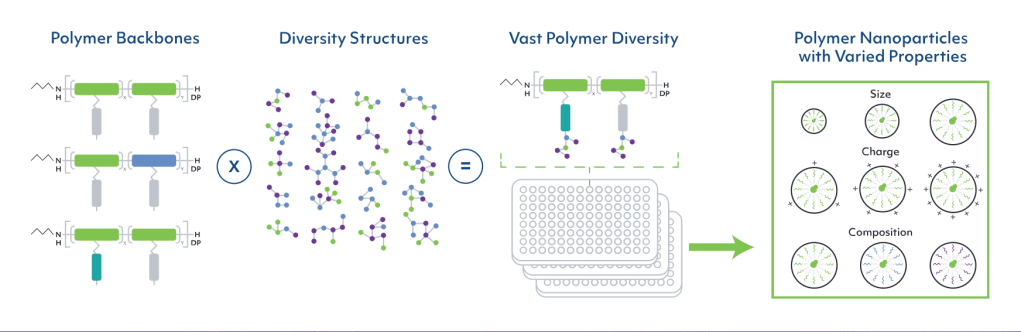
The collaboration, which was announced on Tuesday, aims to develop non-viral, non-lipid, hydrophilic nanoparticles (HNPs) discovered through GenEdit’s NanoGalaxy® platform. NanoGalaxy includes a library consisting of thousands of unique non-viral, non-lipid, hydrophilic polymer nanoparticles.
Using this combinatorial library of polymers, NanoGalaxy creates nanoparticles consisting of hydrophilic polymer backbones with diverse small-molecule side chains, all combined with a variety of structures to yield hundreds of thousands of structurally distinct polymers capable of targeting certain cells. The HNPs derived from these polymers can be further diversified by size and other properties, depending on where in the body they are needed to deliver their payloads.
GenEdit says its technology offers several advantages over other delivery methods, including tissue selectivity, payload flexibility, low immunogenicity, ability to re-dose, and ease of manufacturing. The size of functional payloads ranges from 20-basepair siRNA constructs to 10-kilobase constructs, well beyond the approximately 5 kb size limits associated with adeno-associated viruses (AAVs).
The polymer nanoparticles can encapsulate diverse payloads, according to GenEdit, including nucleic acids such as messenger RNA (mRNA), antisense oligonucleotides (ASOs), or silent RNA (siRNA); therapeutic proteins and CRISPRs.
Autoimmune and inflammatory diseases are within the “immunology” category, one of eight areas where Genentech is pursuing partnerships. (The other seven are cardiovascular and metabolic disease; oncology and cancer immunotherapy; infectious diseases, neuroscience, ophthalmology, research technologies (including “genomic medicines” and “targeted or intracellular delivery”), and digital and personalized healthcare.)
“Genentech and Roche really have a culture of bringing innovation to the clinic, and they are very, very interested in the autoimmune space. The fortunate part is that we are both in the same location, in South San Francisco,” Lee recalled. “While we were in conversation, we found that they were interested in a particular autoimmune application, and they were looking for the right technology. We got connected based on that.”
Roche agreed to pay GenEdit $15 million upfront and up to $629 million in payments tied to achieving near-term, preclinical, and clinical development, commercial, and net sales milestones—plus tiered royalties on global net sales of products developed through the partnership. Genentech will oversee preclinical, clinical, and regulatory development—as well as commercialization of products resulting from the use of GenEdit’s nanoparticles.
“We look for external innovation to complement our internal science to help advance transformative medicines for people living with autoimmune diseases,” James Sabry, global head of Roche Pharma Partnering, said in a statement.
GenEdit is among genetic medicine developers seeking to develop non-viral delivery methods for delivering gene therapies and other genetic medicines, with the aim of avoiding the toxicities seen in clinical trials where patients have received high doses of AAV vectors—the subject of a 2021 FDA advisory committee hearing.
Other companies pursuing non-viral gene therapy delivery that GEN reported last year include:
- Eyevensys uses DNA plasmids encoding therapeutic proteins to treat retinal diseases, such as wet age-related macular degeneration and geographic atrophy.
- Vesigen Therapeutics produces engineered versions of naturally occurring extracellular vesicles called ARMMs (ARRDC1-mediated microvesicles), as some non-viral options, such as lipid nanoparticles, can trigger the immune response as they carry a therapeutic cargo into the cytoplasm.
- CyGenica has engineered a negatively charged cell-penetrating protein that eschews endocytosis.
- And Avectas has developed an ex vivo cell editing technology called Solupore. Inside a closed single-use transfection chamber, cells are engineered by spraying them with a novel solution containing a gene editing cargo as well as an atomizer that creates a transient permeabilization to the cell membrane.
Lee joined Niren Murthy, PhD, now a professor at UC Berkeley, and Hyo Min Park, PhD, to establish GenEdit in 2016, with the goal of establishing safe and effective strategies for delivering genetic treatments. At the time, they referred to their startup as “the UPS for gene editing.”
“We backed Kunwoo and Hyo Min for several reasons,” Bow Capital, a pre-seed investor in GenEdit, stated on its website. “We saw their passion for the space and their immense desire to create true societal good.” Lee is an academic “who very naturally crossed over to become an entrepreneur, which is rare, and something we value.”
Park brought years of valuable lab experience. “Together, they’ve built a sharp technical team with top researchers from all over the country,” Bow Capital observed.
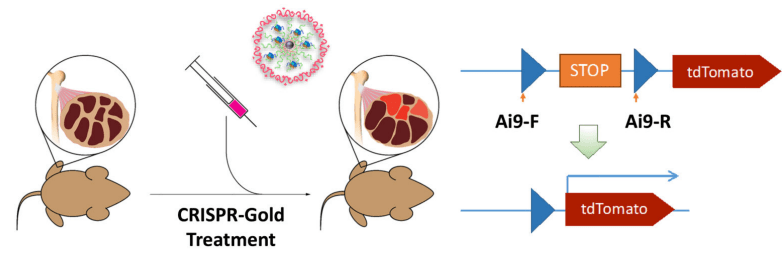
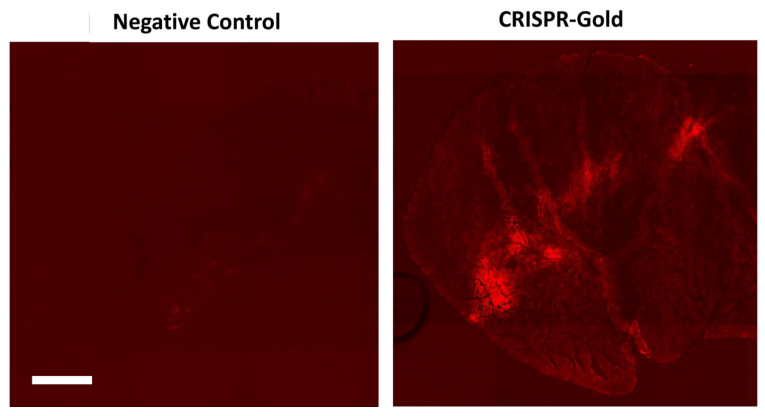
A year later, GenEdit’s co-founders joined Doudna and 18 colleagues in publishing a paper in Nature Biomedical Engineering detailing an early triumph of their research, by reporting that they had engineered a gold nanoparticle technology for delivering CRISPR-Cas9 inside cells.
The researchers also showed in mice that a single injection of the delivery technology, called CRISPR-Gold, could repair the mutation that causes DMD. Mice treated with CRISPR-Gold showed an 18-times-higher correction rate and a two-fold increase in a strength and agility test compared to control groups.
CRISPR-Gold consists of 15-nanometer gold nanoparticles conjugated to thiol-modified oligonucleotides (DNA-Thiol), which were hybridized with single-stranded donor DNA and subsequently complexed with Cas9 and encapsulated by a polymer that disrupted the endosome of the cell. While the delivery technology showed success, with DNA damage similar to that of a typical DNA sequencing error in a typical cell that was not exposed to CRISPR (0.005 – 0.2%), GenEdit was concerned enough about the potential for DNA damage that it switched to gold-free delivery by engineering HNPs.
GenEdit raised an $8.5 million seed round in 2018, followed by completion of a $26-million Series A financing in 2021. GenEdit has raised a total $58.5 million in financing since it was established, including the $24 million in Series A1 financing that GenEdit also announced on Tuesday.
Proceeds from the series A1 are intended to support continued development of the NanoGalaxy platform and a pipeline of preclinical therapeutic candidates. GenEdit has not disclosed individual candidates within its pipeline, although Lee said the company is focusing on developing treatments for CNS disorders and extrahepatic disorders, in addition to the autoimmune therapies it is partnering with Genentech to develop.
The Genentech collaboration is GenEdit’s latest partnership with a biopharma partner. GenEdit launched an alliance with Sarepta in 2020 to develop gene editing therapies for neuromuscular diseases. The companies publicized their alliance and subsequent progress in 2022, with Sarepta paying GenEdit $57 million in near-term payments, plus “significant” payments tied to achieving development, regulatory, and commercial milestones, as well as tiered royalties on future product sales.
GenEdit’s latest financing attracted new investors that included KDB Silicon Valley, Mirae Asset Venture Investment, ACVC Partners, Pathway Partners, LoftyRock Investment, Terra VC, K2 Investment, Dong-A ST, KIMCO and Huons—as well as existing investors Sequoia Capital, Korea Investment Partners, Woori Venture Partners, DAYLI Partners, KB Investment, IMM Investment, TIMEFOLIO Asset Management—and Eli Lilly.
Eli Lilly houses GenEdit among other startups at its Gateway Labs by Lilly, a shared innovation accelerator in South San Francisco. The site is one of two facilities for GenEdit, which also occupies a satellite lab in South Korea. Between the two facilities, GenEdit has a workforce of about 40 people.
“We have been gradually growing over time,” Lee said. “There will be some additional increase in FTEs that we expect for this collaboration we are starting, as well as for the ones that we are doing and currently talking to potential partners as well.”
한국뉴스에서도 기대되는 바이오텍으로 소개되고 있습니다.
“유전자 치료제로 美시장서 우뚝설 것” – 매일경제 2/4/2024
진에딧은 이 대표와 박효민 수석부사장이 UC버클리에서 2016년 함께 설립한 스타트업이다. 진에딧은 2022년 한국에 법인을 설립하고 한국인 연구자들을 채용하고 있다. 박 수석부사장이 한국법인 대표를 겸하고 있다.
홈페이지에 나온 자료를 바탕으로 하면 Polymer backbone에 side chain을 결합시킨 후 만든 Polymer nanoparticle library를 이용해서 screening을 합니다.
Agnostic High Throughput screening을 한 이후에 SAR을 통해서 계속적으로 optimization을 하고 CNS, Lung, Immune Systems를 표적 세포로 해서 연구한다는 개념입니다.
이근우 박사와 박효민 박사의 Entrepreneur 여정이 어떻게 될지 많은 기대가 됩니다.
