안녕하세요 보스턴 임박사입니다.
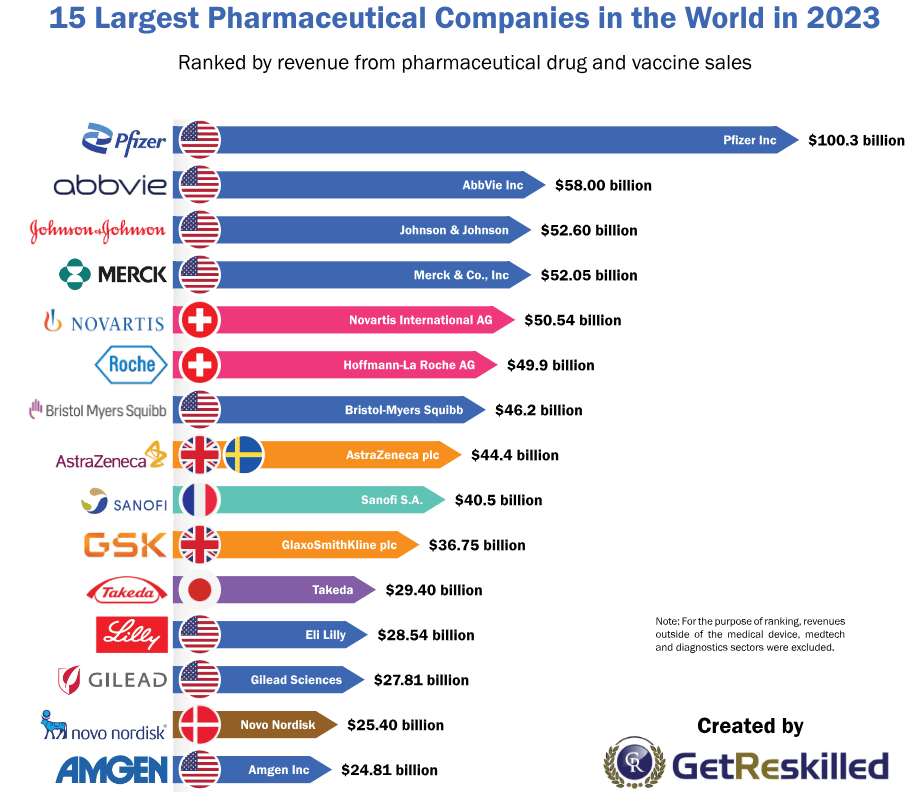
Novo Nordisk는 덴마크에서 Insulin을 생산하는 회사로 1923년에 창업해서 이제 100년이 넘는 역사를 가진 제약회사입니다. 2023년 현재 전세계 Top15 거대제약회사 중의 하나이고 당뇨병 치료제로만 이런 성적을 거두고 있습니다. Novo Nordisk가 최근에 주력하는 약물은 Semaglutide라는 Glucagon-Like Peptide 1 (GLP-1) Receptor에 작용하는 펩타이드 약물로서 주사제인 Wegovy, Ozempic이 있고 경구용 약물인 Rybelsus 이렇게 세가지 품목을 판매하고 있습니다.
- Wegovy: Semaglutide 2.4mg Injection for weight loss – https://www.wegovy.com/
- Ozempic: Semaglutide 0.5mg, 1mg 2mg for Type 2 diabetes – https://www.ozempic.com/
- Rybelsus: Oral Semaglutide Tablet 7mg, 14mg for Type 2 diabetes – https://www.rybelsus.com/
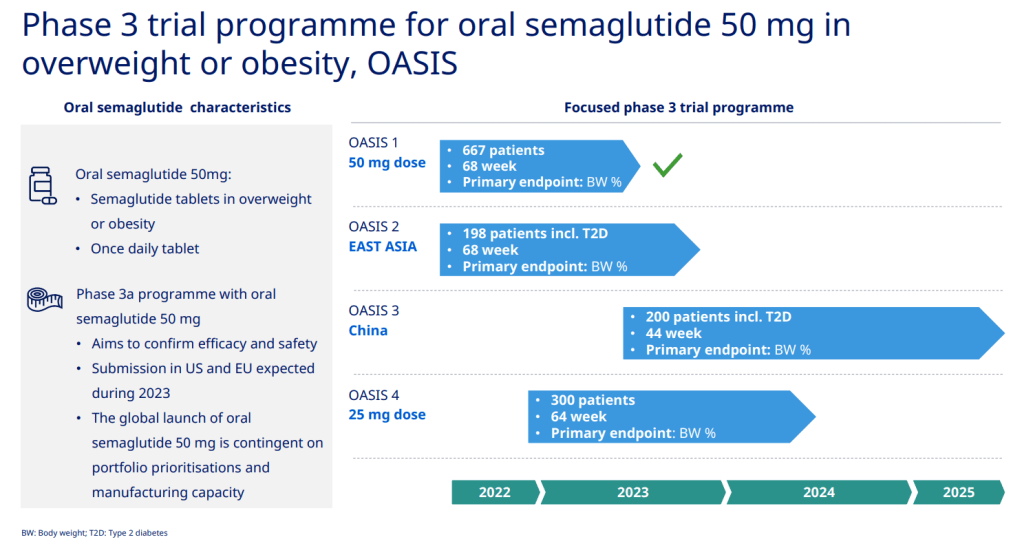
그리고 현재 Weight loss 약으로 50mg oral Semaglutide의 OASIS 임상3상이 진행 중입니다.
Glucagon-Like Peptide-1 (GLP-1)은 31개 아미노산으로 연결된 펩타이드인데 이것의 발견이 있기 전에 Mount Sinai 병원의 John Eng 박사가 한 1990년대 초반의 연구부터 시작을 합니다. John Eng 박사는 남아메리카에 서식하는 도마뱀인 Gila monster의 타액에서 Exetadin-4라는 펩타이드를 발견하게 되는데 이 펩타이드가 혈당을 낮추는 효과가 있다는 것을 발견하게 되고 이 펩타이드와 유사한 인간 펩타이드 GLP-1을 발견하여 세상에 이 사실을 알리게 됩니다.

아래의 논문은 John Eng박사가 1997년에 GLP-1에 대한 연구결과를 Journal of Biological Chemistry에 발표한 논문입니다.
John Eng박사는 이 연구결과를 신약개발에 활용할 수 있다는 확신을 가지고 정부기관, 제약회사 등을 찾아다니며 설득을 했지만 결국 큰 소득을 얻지 못하게 되고 작은 바이오텍인 Amylin Pharmaceuticals에 이 기술을 팔았는데 2005년에 Eli Lilly-Amylin Pharmaceuticals가 BYETTA(TM) (Exenatide) Injection의 FDA 승인을 얻습니다.
Exenatide는 39개의 아미노산으로 구성된 펩타이드인데 주사제로 개발이 되었고요 새로운 당뇨병 치료제의 개발이 필요했습니다. 또한 GLP-1이 당뇨 뿐만 아니라 체중감소에도 효과가 있는 기전인 것이 밝혀지며 Novo Nordisk는 새로운 GLP-1 R Agonist 개발에 뛰어들게 되고 Semaglutide라는 약물을 얻게 됩니다.
John Eng박사의 연구와 Semaglutide의 개발 승인까지의 이야기는 Hilary Brueck이 Business Insider India에서 잘 다뤄주고 있습니다.
What does the Gila monster have that we don’t have? The key to more effortless weight-loss, apparently.
It turns out the venom of a small, Southwestern lizard — the only venomous lizard in America — played a critical role in developing a whole new class of blockbuster anti-obesity drugs, called GLP-1s.
One of the newest GLP-1s is called semaglutide. It’s sold under the brand names Ozempic and Wegovy — and it is taking Hollywood by storm. Rising demand for these types of drugs, which mimic key hormones that tell us to feel full, have led to severe shortages of GLP-1s in recent months.
But before semaglutide became the darling shot of Hollywood, scientists discovered that compounds in the venom of Gila monsters could help drug developers make better diabetes medications than they’d ever had before.
Gila monster hormones can regulate blood sugar very well
It all started back in the early 1990s, when government researcher Dr. John Eng discovered that Gila monsters have a special hormone in their venom. The hormone is quite similar to a hunger-regulating hormone humans harbor in the small intestine, which helps control blood sugar levels.
In people, it’s called glucagon-like peptide-1. In Gila monsters, Eng named it exendin-4.
Exendin-4 degrades more slowly than the human form of GLP-1, lasting for hours instead of minutes. That means it’s a much better model for drug development, since it wouldn’t be practical to take a drug dozens of times a day.
At first, Eng tried to point this remarkable feature of Gila monster spit out to pharmaceutical makers and the government. He shopped his idea around at the Department of Veterans Affairs, where he worked at the time, as well as several different pharmaceutical companies, but didn’t have much success. In the end, he patented the molecule in 1995, and licensed the discovery to a now-defunct biotech startup called Amylin.
Amylin used Eng’s Gila monster research to create a synthetic hormone, called extenatide. Extenatide was approved by the Food and Drug Administration (FDA) in 2005 to treat type 2 diabetes. It’s still used by hundreds of thousands of children and adults with diabetes today.
A safe obesity treatment that slows digestion and curbs cravings
Extenatide was the very first GLP-1-mimicking drug. It ushered in a whole new class of diabetes medications that are arguably safer, and more effective, than previous treatments were. More recently, GLP-1s have been designed to target obesity, too.
Today’s GLP-1s work to help people lose weight because they mimic a hormone our small intestine makes naturally, which regulates hunger in several different key ways.
When a patient’s blood sugar levels are high, GLP-1 drugs send signals to their pancreas to secrete more insulin — but the hormone-mimicking doesn’t stop there. GLP-1s also send signals to a person’s brain, telling their body to feel fuller with less food. Finally, GLP-1s slow down digestion, changing the way a person’s body turns food into energy.
Originally, patients had to take extenatide twice a day. But, over time, newer, more advanced GLP-1s have come to market, with even longer release times (no offense, Gila monsters).
We need animals to create medical breakthroughs like Ozempic, scientist says
Today, most GLP-1s are injected once a day, or just once a week. But they arguably wouldn’t be here if it wasn’t for Eng’s work — which created the very first GLP-1 drug.
In a statement to Insider, Novo Nordisk, the company that makes Wegovy and Ozempic, said that Gila monsters and the discovery of exendin-4 “did not have anything to do with our decision to develop long-acting GLP-1 receptor agonists” for obesity, because “that was based on GLP-1 biology in humans.”
Semaglutide의 개발에 대한 결과는 2015년 Journal of Medicinal Chemistry에 보고를 했습니다. 논문 링크는 아래에 보냅니다. Liraglutide라는 Lead compound로 부터 지속성을 늘리기 위한 다양한 Lead Optimization을 통해 결국 Semaglutide라는 약물을 얻게 되었습니다.
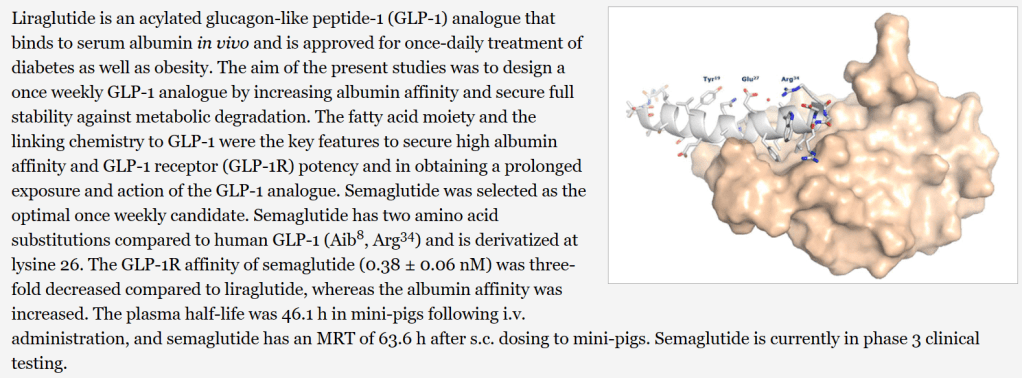
Liraglutide와 Semaglutide의 구조는 아래에서 보이듯이 상당한 Modification이 되었습니다. 세번째 아미노산인 Alanine이 DDP-4에 쉽게 분해되는 것을 막기 위해 Dimethylglycine으로 치환하고 다양한 Lipophilic linker를 집어넣어서 Stability를 크게 늘이는 노력을 기울인 것을 알 수 있습니다.
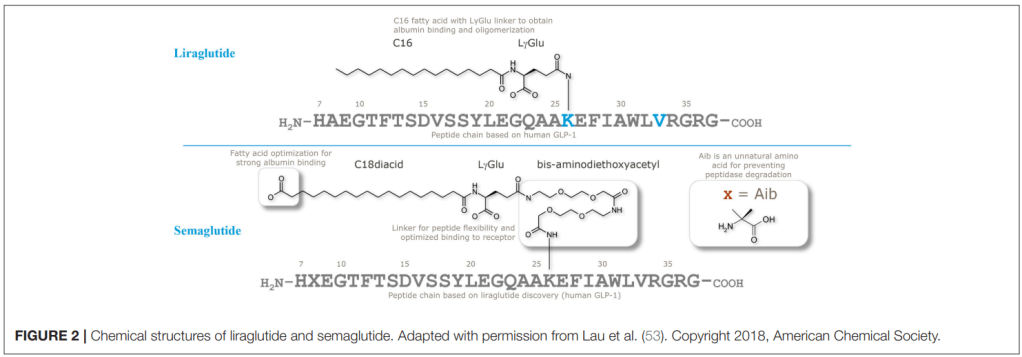
Liraglutide와 Semaglutide의 개발에 대한 스토리는 Novo Nordisk 연구진이 2019년 Frontiers in Endocrinology논문에 비교적 상세히 실었습니다.
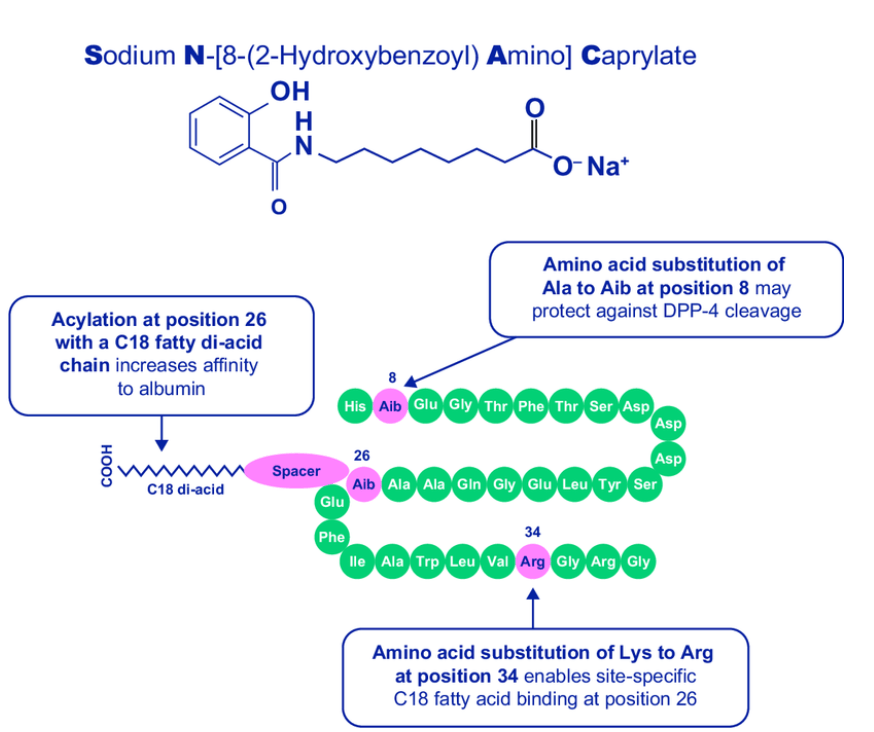
경구용 Semaglutide는 SNAC (Sodium N-(8-[2-Hydroxylbenzoyl] Amino) Caprylate)라는 Absorption Enhancer를 함께 넣어준 새로운 Formulation Drug입니다. SNAC formulation은 본래 Emisphere Technologies가 보유한 기술로서 2007년부터 Novo Nordisk와 공동으로 Oral Semaglutide (Rybelsus) 개발에 사용을 하던 것을 소유권을 얻기 위해 Emisphere를 총 $1.8 Billion에 인수하게 됩니다.
Novo Nordisk A/S today announced that the company has entered into a definitive agreement to acquire Emisphere Technologies Inc. (Emisphere), a drug delivery company with proprietary technologies, such as the Eligen® SNAC technology, that enable oral formulations of therapeutics.
Novo Nordisk and Emisphere have collaborated since 2007 and Emisphere’s proprietary drug delivery technology Eligen® SNAC is used by Novo Nordisk under an existing licence agreement in the oral formulation of Novo Nordisk’s GLP-1 receptor agonist semaglutide, which is marketed and sold under the brand name Rybelsus®.
Under the terms of the agreement, Novo Nordisk will acquire all outstanding shares of Emisphere for USD 1.350 billion. As part of the transaction, Novo Nordisk will also acquire related Eligen® SNAC royalty stream obligations owed to MHR Fund Management LLC (MHR), the largest shareholder of Emisphere, for USD 450 million. Consequently, the total acquisition price is USD 1.8 billion.
With these acquisitions, Novo Nordisk eliminates its future royalty obligations to Emisphere and MHR and obtains full access to the Eligen® SNAC technology platform thereby enabling Novo Nordisk to expand the portfolio of oral biologic pipeline assets across therapy areas.
The transaction will be debt financed and will not impact Novo Nordisk’s previously communicated operating profit outlook for 2020 or the ongoing share buyback programme. The acquisition is expected to have a net negative impact on operating profit of less than one percent in 2021 and broadly neutral to positive impact in the following years.
The acquisition of Emisphere provides Novo Nordisk full ownership of the Eligen® SNAC technology, which has been successfully used under a licence agreement to develop the first oral biologic, Rybelsus®” said Mads Krogsgaard Thomsen, executive vice president and chief scientific officer of Novo Nordisk. “We intend to apply and further develop the technology and use it on current and future pipeline assets with the aim of making more biologic medicines orally available for patients”.
The transaction is subject to customary closing conditions, including approval by Emisphere shareholders and the expiration or termination of the waiting period under the Hart-Scott-Rodino Antitrust Improvements Act of 1976. MHR and certain other shareholders of Emisphere, collectively owning a majority of the Emisphere shares, have agreed to vote their shares in favour of the transaction.
Novo Nordisk is represented by Davis Polk & Wardwell LLP as legal advisor and Evercore as financial advisor.
About Eligen® SNAC Carrier Technology
Eligen® SNAC technology enables drug therapies to be provided in a tablet formulation with an absorption-enhancing excipient. Emisphere created Eligen® SNAC technology, its proprietary oral drug delivery platform, to facilitate the absorption of small and large molecules without altering their chemical form, biological integrity or pharmacological properties. Notably, the technology enables the transport of therapeutic molecules including large peptides and proteins across biological membranes such as those of the gastrointestinal tract.
About Emisphere Emisphere is a drug delivery company that utilises its proprietary technologies to develop new oral formulations of therapeutic agents. For more information, please visit Emisphere’s website at www.emisphere.com.
SNAC oral delivery fomulation 에 대한 좋은 리뷰가 있어서 두개를 올립니다. 첫번째는 영국 University of Wales 논문입니다.
두번째 논문은 University of Texas의 논문으로 SNAC에 대한 리뷰입니다.
Rybelsus (Oral Semaglutide 7mg, 14mg for Type 2 Diabetes)의 임상시험 결과는 New England Journal of Medicine 2019년에 보고했습니다.
그리고 Wegovy (Semaglutide 2.4 mg injection for weight loss)에 대한 임상시험 결과는 2021년 New England Journal of Medicine에 발표를 했습니다.
이런 노력의 결과 Novo Nordisk의 Rybelsus (Oral Semaglutide SNAC formulation: 7mg, 14mg for type 2 diabetes)이 2019년에 FDA로 부터 승인을 받게 되었습니다.
Novo Nordisk today announced that the U.S. Food and Drug Administration (FDA) has approved Rybelsus® (semaglutide) tablets 7 mg or 14 mg for adults with type 2 diabetes that along with diet and exercise may improve blood sugar (glucose).1 Rybelsus® is the first and only glucagon-like peptide-1 (GLP-1) analog in a pill and a new option for adults with type 2 diabetes who are not achieving their A1C goal with current antidiabetic treatment.
Type 2 diabetes is a global public health issue that impacts more than 28 million people in the U.S. alone.2 Despite existing treatment options, many adults with type 2 diabetes have poorly managed blood sugar that can increase the risk of developing serious diabetes-related complications.2-3
“GLP-1 receptor agonists are effective medications for people with type 2 diabetes but have been underutilized in part because they have, until now, only been available as an injectable treatment,” said Vanita R. Aroda, MD, Director of Diabetes Clinical Research, Brigham and Women’s Hospital, Boston, MA and a PIONEER clinical trial investigator. “The availability of an oral GLP-1 receptor agonist represents a significant development and primary care providers, specialists and patients alike may now be more receptive to the use of a GLP-1 therapy to help them achieve their blood sugar goals.”
The approval of Rybelsus® is based on results from 10 PIONEER clinical trials, which enrolled 9,543 participants and included head-to-head studies of Rybelsus® vs. sitagliptin, empagliflozin and liraglutide 1.8 mg.4 In the trials, Rybelsus® reduced A1C and, as a secondary endpoint, showed reductions in body weight. The most common adverse reactions in the PIONEER trials, reported in ≥5% of patients, were nausea, abdominal pain, diarrhea, decreased appetite, vomiting and constipation. The types and frequency of the adverse reactions were similar across trials.1,5-7
“People living with type 2 diabetes deserve more innovation, research and support to help them achieve their individual A1C goals,” said Todd Hobbs, vice president and U.S. chief medical officer of Novo Nordisk. “With Rybelsus®, we have the opportunity to expand use of effective GLP-1 receptor agonist therapy by providing adults with type 2 diabetes an oral medication which was previously only available as an injection to help with managing their blood sugar.”
Rybelsus® is approved for once-daily use in two therapeutic doses, 7 mg and 14 mg, and will be available in the U.S. beginning in Q4 2019. Initial supply of Rybelsus® will come from manufacturing facilities in Denmark; however, future supply for Rybelsus® will come from manufacturing facilities in the U.S. In 2015, Novo Nordisk made a strategic investment to build a new manufacturing facility in Clayton, NC to prepare for the future demand for Rybelsus®. Additionally, earlier this year Novo Nordisk acquired a tableting and packaging facility in Durham, NC to meet anticipated supply needs for Rybelsus®.
Novo Nordisk is working with health insurance providers with a goal of ensuring broad insurance coverage and patient access to the product. A savings card program will be available at the time of launch for eligible commercially-insured patients to keep out of pocket costs down to as little as $10 a month.
The U.S. FDA is still reviewing Novo Nordisk’s new drug application (NDA) for Rybelsus® seeking an additional indication to reduce the risk of major adverse cardiovascular events (MACE) such as heart attack, stroke, or cardiovascular death in adults with type 2 diabetes and established cardiovascular disease (CVD). A decision is expected in Q1 2020.
Rybelsus® is currently under review by several regulatory agencies around the world, including the European Medicines Agency and the Japanese Pharmaceuticals and Medical Devices Agency.
What is Rybelsus®?
Rybelsus® (semaglutide) tablets 7 mg or 14 mg is a prescription medicine for adults with type 2 diabetes that along with diet and exercise may improve blood sugar (glucose).
- Rybelsus® is not recommended as the first choice of medicine for treating diabetes
- It is not known if Rybelsus® can be used in people who have had pancreatitis
- Rybelsus® is not for use in people with type 1 diabetes and people with diabetic ketoacidosis
- It is not known if Rybelsus® is safe and effective for use in children under 18 years of age
그리고 Rybelsus는 first-line type 2 diabetes option으로 FDA label update가 되어 이제 많은 당뇨환자들에게 쓰일 수 있게 되었습니다.
The U.S. Food and Drug Administration (FDA) has approved a label update for Rybelsus® (semaglutide) tablets 7 mg or 14 mg, allowing use as a first-line treatment option for adults with type 2 diabetes who have not previously taken a diabetes treatment. This update removes a previous limitation of use that stated the medication should not be used as the initial therapy for treating patients with type 2 diabetes. Initially approved by the FDA in 2019, Rybelsus® is the first and only GLP-1 analog in pill form and is indicated, along with diet and exercise, to improve glycemic control for adults with type 2 diabetes.1,2
“The removal of the limitation of use is an important step forward for people living with type 2 diabetes and provides the option for Rybelsus® to be taken earlier,” said Dr. Aaron King, Family Medicine and Diabetes Specialist. “By taking Rybelsus® first, people with type 2 diabetes, in conjunction with their care teams, are now able to utilize this medicine early in their diabetes treatment journeys.”
Rybelsus® works differently than other diabetes pills to lower blood sugar in three ways: by increasing the release of insulin from the pancreas when blood sugar is high, decreasing the release of sugar from the liver, and slowing the process of food leaving the stomach after eating.1,2 Rybelsus® comprises a unique co-formulation of semaglutide and an absorption enhancer called SNAC (sodium N-(8-[2-hydroxybenzoyl] amino) caprylate), which facilitates absorption of semaglutide in the stomach, making it possible to provide semaglutide as a pill.4
“In the U.S., hundreds of thousands of people with type 2 diabetes have been prescribed this medicine as part of their type 2 diabetes treatment regimen to help lower their A1C,” said Doug Langa, executive vice president, North America operations and president of Novo Nordisk. “As Novo Nordisk marks 100 years of commitment and innovation in diabetes care, Rybelsus® remains a pivotal part of our portfolio, making history as the first oral GLP-1 receptor agonist and helping to fuel our mission to improve the lives and health of people living with diabetes.”
Novo Nordisk works with health insurance providers to ensure broad insurance coverage and patient access to Rybelsus®. Eligible, commercially insured patients may pay as little as $10 for a one- to three-month prescription of this medicine.
For more information about Rybelsus®, visit Rybelsus.com. For health care professionals, please visit RybelsusPro.com.
What is RYBELSUS®?
RYBELSUS® (semaglutide) tablets 7 mg or 14 mg is a prescription medicine used along with diet and exercise to improve blood sugar (glucose) in adults with type 2 diabetes.
- It is not known if RYBELSUS® can be used in people who have had pancreatitis
- RYBELSUS® is not for use in people with type 1 diabetes
- It is not known if RYBELSUS® is safe and effective for use in children under 18 years of age
2023년 6월에 Novo Nordisk가 ADA (American Diabetes Association)에서 발표한 자료가 아래에 링크했습니다. Semaglutide 이외의 Novo Nordisk 약물에 대한 내용도 함께 있습니다.
현재 Oral Semaglutide SNAC formulation 50mg에 대한 OASIS 임상3상이 진행 중인데 이에 대한 결과는 The Lancet 2003년에 발표했고 아마도 곧 승인되어 Wegovy의 weight loss 주사제로 부터 새로운 경구용 체중감소제의 시장이 곧 열릴 것 같습니다.
30여년전 John Eng 박사의 연구로 부터 시작된 새로운 도마뱀 타액으로 부터 분리한 Exatin-4 peptide의 발견과 Human GLP-1의 발견 그로부터 이제 새로운 당뇨와 비만치료제의 시대가 열리고 있는 것입니다.

One thought on “BIOTECH (119) Novo Nordisk: Semaglutide – Wegovy, Ozempic & Rybelsus for Type 2 Diabetes & Weight Loss”