안녕하세요 보스턴 임박사입니다.
Eli Lilly와 Novo Nordisk의 Oral GLP-1R Agonist를 Type 2 Diabetes 치료제와 비만치료제로서 개발하는 경쟁이 매우 뜨겁습니다. 작년 6월에 열렸던 ADA (미국당뇨학회)에서 Novo Nordisk의 Oral Semaglutide는 15%(68주) 의 체중 감량을 보였고 Eli Lilly의 Orforlipron도 15% (36주)까지의 체중감량을 발표했습니다. 사실상 거의 동일한 결과라고 볼 수 있습니다. 실로 강대강 대결 양상입니다.
The potential advantages of a daily-pill version of popular GLP-1 drugs for Type 2 diabetes and obesity are obvious compared to the weekly injection routine most patients taking these drugs undergo. Last weekend at the American Diabetes Association (ADA) Scientific Sessions in San Diego, market leaders Novo Nordisk and Eli Lilly presented data that showed their investigative oral GLP-1 treatments are making progress.
A phase 3 study of Novo Nordisk’s high-dose oral semaglutide, OASIS 1, shows that obese patients averaged a weight loss of 15% after 68 weeks of treatment, with 34% seeing a 20% drop in their weight. The results are comparable to the weight reductions seen with Novo’s injected GLP-1 drugs.
Also at the ADA conference, Lilly presented results from a phase 2 trial of its weight-loss pill orforlipron, which produced average weight losses of between 9% and 15% depending on the dose provided over a 36-week period. Doses tested were 12 mg, 24 mg, 36 mg and 45 mg. Results were published Friday in The New England Journal of Medicine.
Eli Lilly가 임상2상을 진행 중인 Orforlipron (OWL833/LY350297)는 Roche의 일본 자회사인Chugai Pharmaceutical이 개발한 약물입니다.
Chugai 제약과 Eli Lilly 연구원은 2020년 PNAS 논문에 Orforlipron (OWL833/LY350297)의 개발에 대해 발표했습니다.
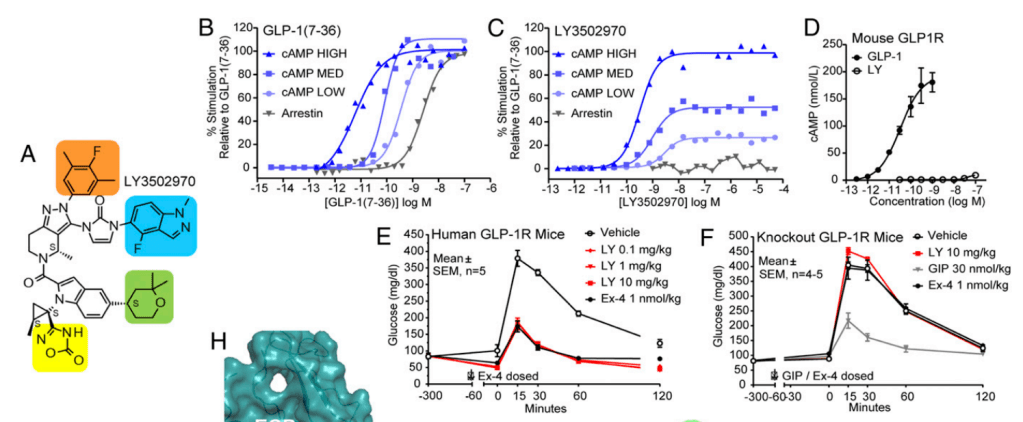
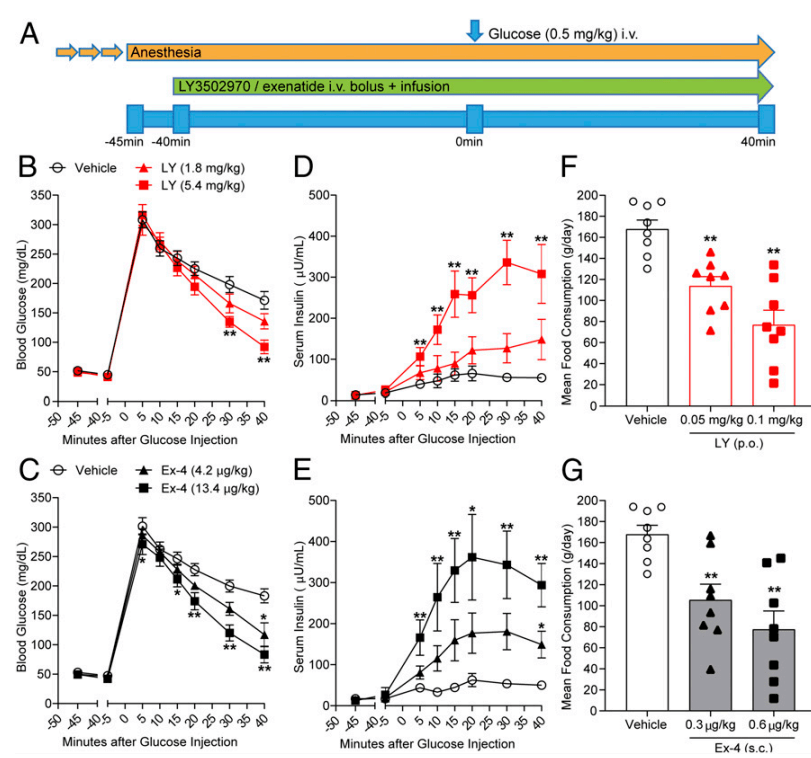
Cyno Molgus를 이용한 NHP Study 결과는 다음과 같습니다. Exenatide i.v. 주사제와 비교한 실험에서 대등한 결과를 보여주어서 Orforlipron (OWL833/LY350297) 경구투여제의 임상시험을 지지했습니다.
Eli Lilly는 Orforlipron (OWL833/LY350297)를 $50 Million upfront를 포함한 마일스톤과 로열티 계약을 Chugai 제약과 체결함으로써 이 약물을 확보하게 됩니다. 2018년 당시에는 아직 전임상 단계였습니다.
Eli Lilly nets an early-stage GLP-1 diabetes drug from Chugai for $50M – Fierce Biotech 9/27/2018
Eli Lilly & Co. is looking to pad out its diabetes portfolio by licensing an oral, non-peptidic GLP-1 receptor agonist from Chugai Pharmaceutical that the company describes as a “phase 1 ready” asset for the treatment of Type 2 disease.
In return for $50 million upfront, Lilly will receive worldwide development and commercialization rights to OWL833, with Chugai, a Tokyo-based member of the Roche Group, eligible for future milestone payments and royalties.
“We believe OWL833 can be a best-in-class oral non-peptide GLP-1 receptor agonist and that its value will be further enhanced through Lilly’s clinical development to contribute to people around the world who live with diabetes,” Yasushi Ito, M.D., Ph.D., Chugai’s executive VP and co-head of its Project & Lifecycle Management Unit, said in a statement.
Both companies said there would be no changes to their financial forecasts or guidances for 2018 as a result of the deal, and that OWL833 will “soon” enter phase 1 clinical development.
Lilly has been working to grow and broaden the technologies in its diabetes pipeline, as well as extend its returns with stronger forays into devices and real-world evidence generation.
In April, the company paid $63 million for Type 1 diabetes cell therapies from Sigilon Therapeutics, and signed on for an additional $410 million in possible milestone payments.
The Cambridge, Massachusetts-based Sigilon aims to induce pluripotent stem cells become insulin-producing beta cells, encapsulated using its Afibromer islet cell technology to protect the implanted cells from the immune system.
A few months prior, Lilly unveiled a device-driven strategy to weather pricing pressures, following its partnership with Dexcom to develop continuous glucose monitoring systems. Lilly has also been working on an automated, wearable insulin delivery device and smart pen injector at a small lab it launched in 2015.
2023년 New England Journal of Medicine에 Orforglipron의 임상2상 결과를 발표했습니다.
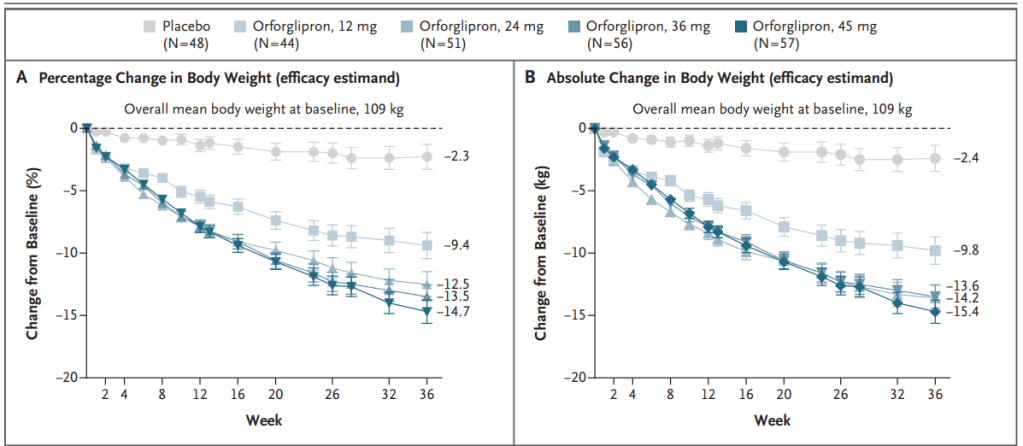
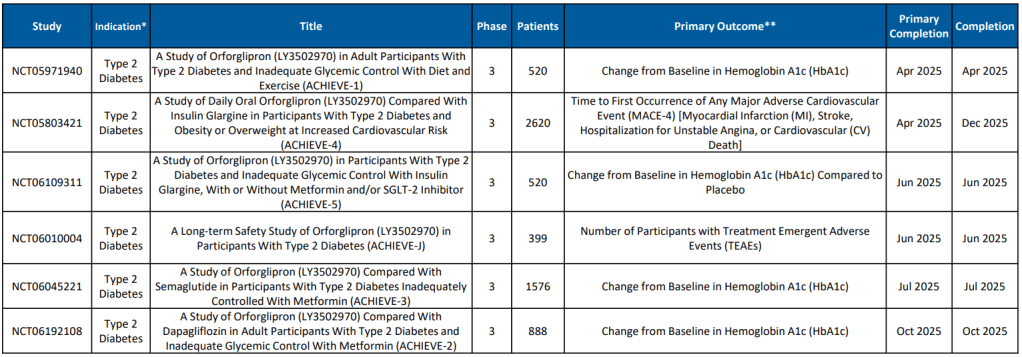
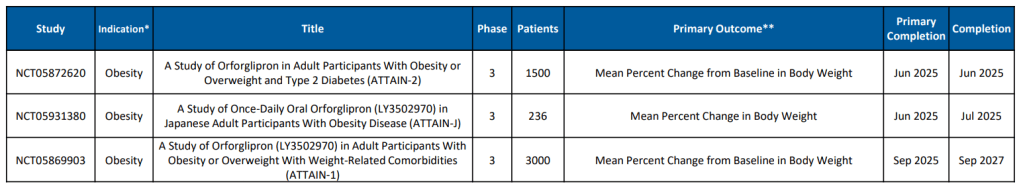
Eli Lilly에서 올해 2월에 발표한 자료에 의하면 Orforglipron에 대한 9개의 임상3상이 진행 중에있습니다.
Non-peptide GLP-1R Agonist로 Pfizer의 Danuglipron이 twice-a-day oral GLP-1R Agonist 약물로 개발 중이었지만 최근에 독성문제로 인해 임상이 중단되었습니다. 대신에 once-a-day oral formualtion의 PK data를 통해 새로운 임상이 시작될 수 있슴을 얘기했습니다. Pfizer는 oral GLP-1 pill의 시장규모를 $30 Billion으로 예상하고 있습니다.
Pfizer plans for oral obesity drug hit new setback – Biopharmadive 12/1/2023
Pfizer’s drug, known as danuglipron, is a GLP-1 agonist like in-demand obesity treatments from Novo Nordisk and Eli Lilly. But unlike those drugs, which are injections, danuglipron is taken orally, an advantage in convenience that Pfizer hopes will help it break into the fast-growing market.
Pfizer didn’t break out adverse event rates by danuglipron dose tested, but said up to 73% of participants experienced nausea, up to 47% vomiting and up to 25% diarrhea. The side effects were generally mild, the company said.
“We believe an improved once-daily formulation of danuglipron could play an important role in the obesity treatment paradigm, and we will focus our efforts on gathering the data to understand its potential profile,” said Mikael Dolsten, Pfizer’s top scientist and head of R&D, in the company’s statement.
Results from the ongoing pharmacokinetic trial of the once-daily version will “inform a potential path forward,” Dolsten added.
Another setback could put Pfizer in the uncomfortable position of falling further behind in a fiercely competitive development race. Lilly, which recently won U.S. approval of its GLP-1 weight loss drug Zepbound, is also developing an oral obesity medicine called oforglipron that showed promise in a mid-stage trial earlier this year.
Pfizer’s CEO Albert Bourla has said he expects the market for GLP-1 drugs, which are also used to treat Type 2 diabetes, to eventually reach $90 billion in sales. He estimated oral versions could claim about one-third of that figure.
Chugai-Eli Lilly가 개발 중인 Orforglipron이 임상 3상을 성공적으로 마치고 승인된다면 Novo Nordisk의 Oral Semaglutide와 함께 Type 2 Diabetes 환자들과 Obesity 환자들에게 큰 도움을 줄 것으로 기대합니다.
