Bicycle Therapeutics는 Phage Display 연구로 2018년에 노벨상을 수상한 Sir. Greg Winter와 Christian Heinis 교수가 개발한 Bicyclic Peptides를 임상에 적용하기 위해 2009년에 설립된 회사입니다.

(Picture: Sir Greg Winter)

2009년 Nature Chemical Biology에 Bicyclic Peptides를 만드는 Phase-Encoded Combinatorial Chemical Libraries를 발표했습니다.
2009년에 회사를 설립한지 5년이 지난 2014년에 $32 Million Series A를 받았습니다.
Bicycle Therapeutics, a next generation biotherapeutics company developing first-in-class bicyclic peptides, today announced that it had secured an equity financing of £20 ($32M) million for the clinical development of therapeutic bicycle drug candidates in oncology. The existing investor syndicate, Atlas Venture, Novartis Venture Fund, SR One, SV Life Sciences and Astellas Venture Management participated in the round.
Bicycle has used its proprietary bicyclic peptide technology that enables it to discover a new class of drug candidates, which have similar selectivity and potency to antibodies but are 100-fold smaller and are manufactured using simple, economic chemical synthesis. This financing will support the clinical development of bicycle-drug conjugates (BDCs) that are highly selective to tumour-specific targets, with sub-nanomolar affinities. Preclinical models show that these BDCs extravasate and penetrate tissues much more rapidly and efficiently than antibodies, effecting rapid cell killing through the tumour mass and fast clearance. This results in effective tumour lysis with minimal systemic exposure.
Bicycle technology has broad applicability in oncology, respiratory, inflammatory and ophthalmology disease, as agonists, antagonists or for delivering payloads. The company’s projects in BDCs for oncology will be leveraged by pharma partnerships addressing targets nominated by partners, where the rapidity of lead discovery and optimisation enables drug candidate selection in months. The first partnership, with ThromboGenics, is developing bicyclic peptide drug candidates to a specific ophthalmology target, for the treatment of diseases such as diabetic macular edema.
Andrew Sandham, Chairman of Bicycle, said, “This second round financing enables us to advance our BDC candidates to clinical development in cancer indications. We also have the capacity to work collaboratively with pharma partners on other targets and indications in many diseases.”
Rolf Günther, CEO, added, “Bicycle technology was invented by our founders, Sir Gregory Winter and Professor Christian Heinis. We have developed the platform for rapid discovery and optimisation of drug candidates, and are delighted that we are now able to demonstrate clinical utility of this exciting new class of molecules.”
2017년에 $52 Million Series B를 했습니다. 이 당시에는 BT1718의 임상을 시작한다는 발표를 했습니다.
Bicycle Therapeutics, a biotechnology company pioneering a new class of therapeutics based on its proprietary bicyclic peptide (Bicycle®) product platform, today announced the successful completion of a £40million Series B financing round. Proceeds will be used to further the development of multiple drug candidates, including Bicycle’s lead molecule, BT1718, a first-in-class drug for cancers of high unmet need.

New investor Vertex Ventures HC led the financing round with participation by additional new investors Cambridge Innovation Capital (CIC) and Longwood Fund. Bicycle’s existing investors – Novartis Venture Fund, SROne, SVLS and Atlas Venture also participated. As part of this financing, the company also announced the addition of Dr. Christopher Shen, M.D., Managing Director at Vertex Ventures HC, and Dr. Michael Anstey, D.Phil., Investment Director at CIC, to its Board of Directors.
“Bicycle Therapeutics has a highly innovative platform with the potential to transform the course of treatment for patients suffering from a range of diseases, including difficult-to-treat cancers,” said Dr. Christopher Shen from Vertex Ventures HC. “We are delighted to lead this financing and to support Bicycle’s seasoned management team to realize the promise of this new class of therapies.”
Bicycle Therapeutics is developing novel first-in-class medicines based on its Bicycle®product platform. Bicycles®can combine properties of several therapeutic entities in a single modality: exhibiting the affinity and selective pharmacology associated with antibodies; the distribution kinetics of small molecules, allowing rapid tumour penetration; and the “tuneable” pharmacokinetic half-life and renal clearance of peptides.
Bicycle’s lead molecule, BT1718, is the first example of its Bicycle Drug Conjugate® (BDC) technology, in which toxic chemical payloads are targeted specifically to malignant tumours, minimising systemic toxin exposure through renal clearance. BT1718 targets Membrane Type 1 Matrix Metalloproteinase (MT1-MTP), which is highly expressed in many solid tumours, including triple negative breast cancer and non-small cell lung cancer. It is expected to enter the clinic in 2017 in partnership with Cancer Research UK (CRUK). The Series B will also fund additional pipeline programs through early clinical development, the first of which will be selected in the second half of 2017.
“This financing represents an important validation of our approach, while providing Bicycle with the resources to continue to advance our pipeline and translate our bicyclic peptide technology into important new treatment options for patients,” said Dr. Kevin Lee, Ph.D., Chief Executive Officer of Bicycle Therapeutics. “We are grateful for the continued strong support from our investors as we move BT1718 rapidly toward the clinic and continue to advance our preclinical programs, including toxin drug conjugates and immune modulators to treat cancer and other debilitating diseases.”
2019년에는 $60 Million Nasdaq IPO를 했습니다. BT1718은 phase 1/2a를 진행 중이었습니다.
Bicycle Therapeutics Expects to Raise $60.6 Million in IPO – Biospace 5/23/2019
Bicycle Therapeutics announced the pricing of its initial public offering (IPO), offering 4,333,333 shares at an IPO price of $14 per share. The company expects to raise about $60.6 million. It is trading on the Nasdaq under the BCYC ticker symbol.
The company focuses on developing a novel class of drugs called Bicycles. Bicycles are fully synthetic short peptides constrained to form two loops—hence “bi” cycles—that stabilize their structural geometry.
The company was founded in 2009 based on science coming out of the laboratory of Sir Greg Winter, winner of the Nobel Prize in Chemistry in 2018 for his work in phage display. Phages are viruses that infect bacteria. The company is co-headquartered in Lexington, Mass. and Cambridge, UK.
On May 7, Bicycle announced a collaboration with the Dementia Discovery Fund (DDF) to use Bicycle technology to develop novel drugs for neurodegenerative diseases. DDF is a specialized venture capital fund focused on discovering and developing therapies for dementia.
“This is a landmark collaboration for Bicycle,” stated Kevin Lee, chief executive officer of Bicycle Therapeutics. “Fifty million people worldwide live with dementia, yet there is no cure for these terrible diseases. Despite the huge burden of these illnesses on individuals, families and society, conventional approaches have so far provided limited benefit. We believe our Bicycles bring a fresh approach to this area, and we are thrilled to work with DDF to apply our proprietary technology to the potential treatment of dementia.”
Under the collaboration, Bicycle will identify Bicycles that bind to clinically-validated dementia targets. If any good compounds are identified, Bicycle will own the resulting intellectual property. Bicycle and DDF will have the option to found a new company together to develop those compounds.
The company’s phage display screening platform is used to identify Bicycles efficiently. Its internal programs focus on cancer. Its lead product candidate is BT1718, a Bicycle Toxin Conjugate in a Phase I/IIa clinical trial funded by the Centre for Drug Development of Cancer Research UK.
BT1718 is being studied for tumors that express membrane Type 1 matrix metalloprotease. The bicycle attaches to the cancer cells and the toxin cleaves from the bicycle and kills the tumor cells. Preliminary data from the trial is expected in the second half of this year.
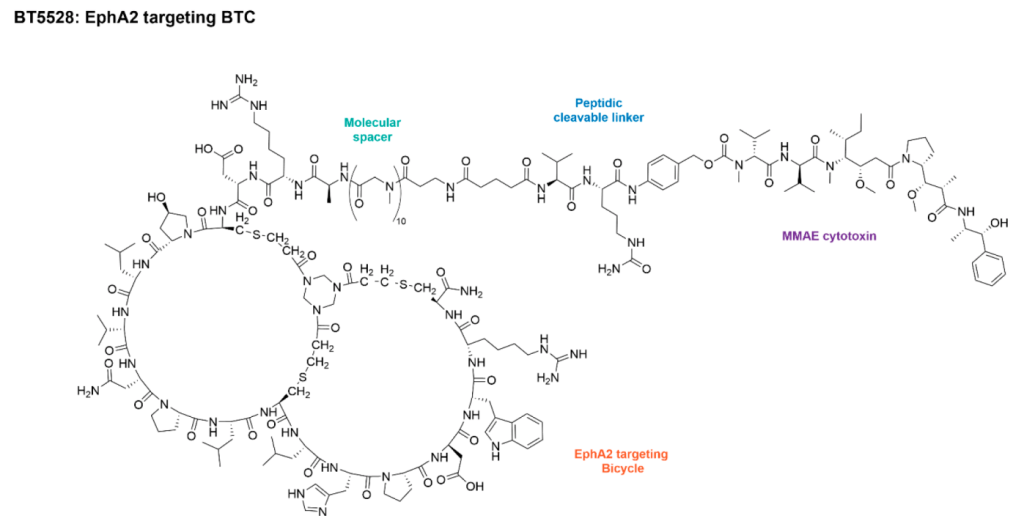
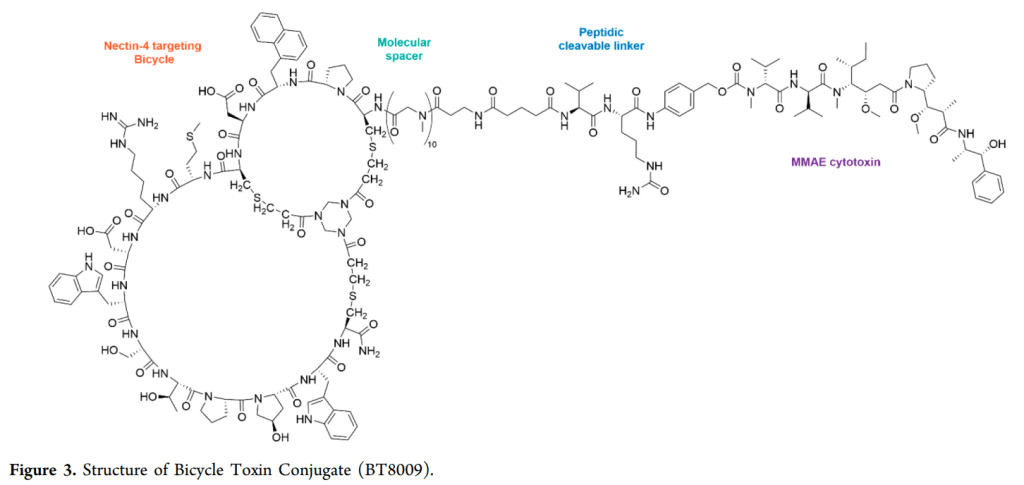
The company’s other pipeline candidates are BT5528 and BT8009, also for oncology indications.
At the end of 2018, Bicycle reported collaboration revenues of $7.14 million. The net loss was $16.3 million for the year, not unusual as operating expenses grew as the company moved its compounds into the clinic.
The company plans to use between $35 million and $40 million of the funds raised for Phase II and Phase III trials of BT1718. It also will use funds to advance its preclinical compounds into the clinic.
Prior to the IPO, the company had raised about $116 million. In addition to the DDF collaboration, Bicycle Therapeutics has cut deals with AstraZeneca to focus on respiratory, cardiovascular and metabolic diseases, with Bioverativ on hemophilia drugs, and with Oxurion on ophthalmology therapeutics.
2024년 2월 현재 파이프라인은 아래와 같습니다. BT8009가 현재 임상3상에 진입해서 pivotal clinical study를 진행하고 있습니다.

