
(Picture: Robert A. Copeland)
안녕하세요 보스턴 임박사입니다.
RNA-level의 Gene Regulation을 Small molecule로 하는 회사들 중에서 Expansion Therapeutics에 대해 글을 쓴 적이 있습니다. Expansion 의 경우는 RNA-Small Molecule Direct Binding에 의해 단백질 합성을 조절하는 메카니즘입니다.
BIOTECH (71) – Expansion Therapeutics – RIBOTAC Degrader by RNA-Small Molecule Binding
Accent Therapeutics는 RNA-Modifying Proteins (RMPs)를 표적함으로써 RNA level Gene Regulation을 하려는 회사로서 2018년에 Epizyme founder이자 CEO 였던 Robert Copeland 박사가 CSO이면서 Stanford University의 Howard Chang 교수와 University of Chicago의 Chuan He 교수와 함께 공동으로 창업을 한 후 시리즈 A $40 Million을 받았습니다.
Accent Therapeutics, a biopharmaceutical company developing breakthrough treatments for cancer patients, today announced $40 million in Series A capital to establish a discovery platform and pipeline of therapeutic candidates targeting RNA-modifying proteins (RMPs), a novel target space for precision cancer therapies. The Column Group, Atlas Venture and EcoR1 Capital provided the investment.
Accent Therapeutics was established to create innovative therapeutics in the rapidly advancing area of epitranscriptomics – the role of RNA structure, stability, function and translation in cell biology. Recent studies have linked certain human cancers to the activity of particular RMPs, providing a rich new target space for drug development. The Accent Therapeutics team includes seasoned drug developers, with an established record of translating novel science into innovative therapies. Leaders of that team have recently published a peer-reviewed overview of advances in the field in Nature Reviews Drug Discovery entitled “RNA-Modifying Proteins as Anticancer Drug Targets” (doi:10.1038/nrd.2018.71).
Accent’s founders include Howard Y. Chang, M.D., Ph.D. of Stanford University, Chuan He, Ph.D. of the University of Chicago and Robert A. Copeland, Ph.D., President and Chief Scientific Officer of Accent Therapeutics, who together bring broad and deep expertise in the emerging biology of epitranscriptomics, its role in human diseases and the translation of novel science to cancer drug discovery and development. “Epitranscriptomics opens a rich new target space, including RMPs that are associated with specific cancers, many with poor patient prognoses,” said Dr. Copeland. “We plan to treat patients by precisely targeting cancers that are uniquely dependent on these specific RMPs.”


“There is great value in targeting the molecular mechanisms that can go awry and drive specific cancers. We are excited to support Accent as they develop efficacious and truly differentiated cancer therapeutics,” said Larry Lasky, PhD., Partner at The Column Group and a member of the Accent Therapeutics board of directors.
“The Accent team is anchored by experienced drug developers with a track record of success in the creation of innovative precision therapies. They are well-suited to undertake the development of effective new therapies targeting RMPs,” said Jason Rhodes, Partner at Atlas Venture and a member of the Accent Therapeutics board of directors.
2년 후에 METTL3와 ADAR1 Programs을 발굴하면서 $63 Million Series B를 했는데 Abbvie Ventures와 Google Ventures 등이 새로 참여를 했습니다.
Accent Therapeutics, a biopharmaceutical company developing breakthrough treatments for cancer patients, announced today that it has completed a $63 million Series B financing. The Series B was led by EcoR1 Capital with participation by GV, AbbVie Ventures, The Mark Foundation for Cancer Research, NS Investment and Droia Ventures as well as existing investors, Atlas Venture and The Column Group.
Proceeds from the financing will be used to advance the development of Accent’s novel therapies targeting RNA-modifying proteins (RMPs), including its lead programs METTL3 and ADAR1, and to continue to expand its pipeline in the rich target space of RNA modification.
“We are thrilled to have the support of this remarkable group of investors that share our vision for developing novel therapies for patients in need,” said Shakti Narayan, Chief Executive Officer of Accent Therapeutics. “With the progress we have made to-date and expect to make in the coming months, the next phase of Accent’s growth is set to be truly transformational.”
Since launching in 2018, Accent has advanced a broad pipeline of programs, including its two lead programs – METTL3 and ADAR1. METTL3 is an RNA methyltransferase implicated in AML, specific solid tumors and immuno-oncology. ADAR1 is an RNA editor with compelling validation for solid tumors with elevated intrinsic Type I interferon-stimulated gene signaling (comprising ~15-30% of solid tumors) and has also been suggested to play a key role in immuno-oncology. By targeting the proteins that modify RNA, Accent is able to apply the proven approach of enzyme-directed small molecule therapies to a rich and novel class of enzymes with the ability to impact RNA pathobiology.
“Opportunities to have such a broad impact in novel areas of biology are becoming increasingly rare,” said Oleg Nodelman, Founder and Managing Director of EcoR1 Capital. “The team at Accent is well-positioned to lead this area of drug development and achieve the rich therapeutic potential of these exciting programs.”
시리즈B를 한 지 몇달 안되어 AstraZeneca와 $55 Million Upfront를 포함 총 $1.1 Billion 규모의 공동연구계약을 했습니다. Accent는 전임상부터 임상1상까지를 맡고 AstraZeneca는 그 이후부터 상용화까지를 맡는 계약이었습니다.
AstraZeneca will partner with Accent Therapeutics to discover, develop, and commercialize cancer therapeutics based on Accent’s RNA-modifying protein (RMP) inhibition technology, the companies said today, through a collaboration that could generate more than $1.1 billion for the Lexington, MA, biopharma.
The partnership is intended to combine AstraZeneca’s expertise in oncology drug development with Accent’s focus on small-molecule, RMP-targeting precision cancer therapeutics. Since its launch in 2018, Accent has developed two lead candidates: METTL3 is an RNA methyltransferase implicated in AML, specific solid tumors, and immuno-oncology; and ADAR1, an RNA editor with compelling validation for solid tumors with elevated intrinsic Type I interferon-stimulated gene signaling.
By targeting proteins that modify RNA, Accent reasons, it can effectively apply the proven approach of enzyme-directed small molecule therapies to a new class of enzymes with the ability to impact RNA pathobiology.
Under its collaboration with AstraZeneca, Accent has agreed to oversee R&D activity for a nominated preclinical program through Phase I clinical trials. Upon completion of Phase I, AstraZeneca has agreed to lead development and commercialization activities for the nominated program, with Accent having the option to jointly develop and commercialize with AstraZeneca in the U.S.
AstraZeneca will also have the exclusive option to license worldwide rights to two further preclinical discovery programs, for which Accent will conduct certain preclinical activities.
“A compelling area”
“The promise of RMP inhibition is a compelling area of exploration for AstraZeneca. With this collaboration, we will seek to identify novel targets and unlock the full potential of our medicines,” José Baselga, executive vice president, Oncology R&D, AstraZeneca, said in a statement.
AstraZeneca has agreed to pay Accent $55 million upfront. If Accent elects to jointly develop the nominated program, AstraZeneca would pay Accent an additional up to $1.1 billion in option fees and payments tied to achieving milestones across all programs, as well as tiered royalties on net sales ranging from mid-single digit to low-double digits.
Both companies agreed to split profits and losses in the U.S.
“This collaborative effort will enable us to rapidly advance and achieve the rich therapeutic potential of these exciting programs,” added Accent CEO Shakti Narayan, PhD, JD. “This collaboration leverages both AstraZeneca’s vast cancer expertise and resources and Accent’s rich pipeline of RMP therapeutic programs to bring new and potentially life-changing medicines to patients.
Accent’s collaboration with AstraZeneca comes less than two months after Accent completed a $63 million Series B financing.
The Series B was led by EcoR1 Capital with participation by GV, AbbVie Ventures, The Mark Foundation for Cancer Research, NS Investment and Droia Ventures as well as existing investors, Atlas Venture and The Column Group.
At the time, Accent said proceeds from the financing would be used to advance its development of RMP-inhibiting therapeutics, including METTL3 and ADAR1, and to continue expanding expand its RNA modification pipeline.
1년 후에는 Ipsen에서 METTL3 프로그램을 총 $446 Million 및 Sales royalties를 포함하는 계약으로 인수했습니다.
Ipsen (Euronext: IPN; ADR: IPSEY) and Accent Therapeutics (Accent) have signed an exclusive worldwide-collaboration agreement to research, develop, manufacture, and commercialize Accent’s pre-clinical stage METTL3 program.
Acute myeloid leukemia (AML) is a difficult to treat cancer of the blood and bone marrow, accounting for a third of all new cases of leukemia in the US each year.1 Globally, the incidence of AML has been increasing year on year across the last 20 years.2 RNA modifying proteins (RMPs) are an emerging target class that control multiple aspects of RNA biology and represent a new approach for the potential treatment of various cancers. METTL3 is an RMP that has been validated pre-clinically as a novel therapeutic target for AML.1,3 This collaboration combines Accent’s expertise in RMP-targeting therapeutics with Ipsen’s capabilities and proven track record in Oncology medicine development and commercialization.
Christelle Huguet, Senior Vice President, Head of Research, External Innovation and Early Development, Ipsen, said “Oncology is a key focus area for Ipsen as we grow our pipeline. We are delighted to partner with Accent to progress the METTL3 program as we continue our expansion into hematologic oncology. Our teams are steadfast in our commitment to areas of high unmet medical need including rare cancers, so this collaboration is strongly aligned with Ipsen’s mission and strategy for growth.”
Shakti Narayan, Chief Executive Officer of Accent Therapeutics said “This collaboration blends Ipsen’s commitment to developing and commercializing transformative oncology medicines with Accent’s leading expertise in the field of RNA modification. As we focus on developing our rich pipeline of novel RMP-targeted therapies, we are pleased to entrust our METTL3 program to the innovative team at Ipsen to bring this novel investigational therapy to patients in need.”
Under the agreement, Ipsen will pay up to $446m, comprising upfront payment as well as pre-clinical, clinical, regulatory, and sales-based milestone payments, plus tiered sales royalties ranging from mid-single digits to low-double digits.
2023년말에 Precision Medicine Online을 통해 그동안의 Accent Therapeutics의 프로그램에 대해 전체적으로 정리하는 기사가 있었습니다.
설립 초기부터 RNA modification을 하는 수백개의 유전자를 발굴하고 이 중에서 항암효과를 가진 유전자를 Knock-out 방법으로 발굴해서 1,500개의 RNA-binding protein을 찾았고 이 중에서 Precision Oncology Targets로 가장 좋은 10여개의 단백질 표적을 발굴했습니다.
Lead Program은 DHX9인데 Colorectal cancer를 포함해서 암치료제로서의 가능성에 대해 2024년 후반에 IND Filing을 하고 2025년에 임상을 시작한다는 계획입니다.
전임상 단계인 ADAR1 프로그램은 Checkpoint Inhibitors에 듣지 않는 Head and Neck Cancer, NSCLC 환자를 대상으로 하고 초기연구 중인 RNA exonuclease XRN1과 또다른 전임상 프로그램으로 Ovarian, Triple-Negative Breast, Small Cell Lung, Colorectal Cancer 환자들을 위한 표적 프로그램이 있습니다. 또한 Ipsen에 Lience-Out한 METTL3은 Acute Myeloid Leukemia 프로그램입니다.
Accent Therapeutics is advancing the first of its pipeline drugs targeting RNA-modifying proteins into investigational new drug (IND)-enabling studies in a range of cancers including tumors with microsatellite instability (MSI-high).
Accent’s lead small molecule therapeutic program inhibits DHX9, an RNA helicase that binds and unwinds double-stranded RNA and DNA. It stabilizes the genome through regulation of cellular processes such as DNA replication, transcription, translation, and RNA processing and transport. Loss of DHX9 interferes with DNA replication and increases genome instability.
Accent Founder and CSO Robert Copeland explained that DHX9’s role in stabilizing the genome is what could make DHX9 inhibitors effective against MSI-high cancers, which comprise a subset of colorectal, uterine, gastric, and other malignancies. The Lexington, Massachusetts-based company is expecting to submit an investigational new drug application for the anti-DHX9 drug in the second half of 2024 and begin clinical trials in late 2024 or early 2025.
“To the best of our knowledge, we’re the only company that has small molecule inhibitors of [DHX9] and is pursuing that as a precision cancer therapeutic,” Copeland said.
Accent launched in 2018 with $40 million in Series A financing and secured another $63 million in a Series B round in April 2020. The company’s mission is to discover and develop therapeutics targeting modifications in post-transcriptional RNA — a new field analogous to epigenetics known as epitranscriptomics. These modifications affect gene expression through regulation of RNA stability, localization, and functional efficiency. In cancer, disruption of the epitranscriptome has been implicated as a driver of tumor growth and drug resistance.
Copeland founded Accent with Howard Chang, a professor of cancer genomics at Stanford University, and Chuan He, a professor of biochemistry at the University of Chicago. Before Accent, Copeland was a founder and CSO at Epizyme, where Copeland guided the development of Tazverik (tazemetostat), an inhibitor of the histone-modifying protein EZH2 approved in the US for non-Hodgkin lymphoma and epithelial sarcoma, and numerous other clinical programs involving proteins that modify DNA.
“In 2017, I was approached by the Column Group about starting a new company that would be focused not on chromatin and histone modifications but instead RNA modifications,” Copeland said. However, Copeland added, “it’s very difficult to have potent selective small molecules that will bind directly to RNA.”
Instead, Accent’s approach is to target the proteins involved in modifying RNA. “It’s a novel area of medicine in targeting RNA pathobiology, [but] we’re doing it through a very well-established mechanism of small molecules that inhibit enzyme targets,” Copeland said.
Accent is particularly focused on cancer, he noted, because that’s the area with the most extensive literature on the effects of RNA modification. Early in the company’s history, Accent researchers studied the human genome and identified hundreds of genes involved in RNA modification. They began systematically assessing the effects of knocking out those genes in cancer cells, looking for a promising anti-cancer result.
Those efforts produced about 1,500 candidate RNA-binding proteins, and from that the researchers picked out about a dozen that were attractive as precision oncology targets, Copeland said. A subset of those became discovery targets for small molecule inhibitors including the preclinical-stage ADAR1 program in head and neck cancer, non-small cell lung cancer, and tumors refractory to checkpoint inhibitors; a discovery-stage program targeting the RNA exonuclease XRN1 in the same set of cancers; and an undisclosed preclinical program in ovarian, triple-negative breast, small cell lung, and colorectal cancers. Accent also licensed a program targeting METTL3 to Ipsen in 2021 for R&D, manufacturing, and commercialization in acute myeloid leukemia.
However, DHX9, as Accent’s most advanced in-house program, made an attractive target, Copeland said, because it is “absolutely essential” for survival of cancers characterized by deficiencies such as DNA damage repair. At the same time, in normal cells, Accent has found that DHX9 is dispensable.
“We can define which cancers are likely to respond to DHX9 knockdown or inhibition with a small molecule, and in that way, identify patients that are most likely to benefit,” Copeland said. Accent has selected a lead drug candidate and begun investigational new drug application-enabling studies with the intention of submitting an application in the second half of 2024, which if cleared by the FDA will allow it to take the agent into clinical trials.
In preclinical studies, targeting DHX9 was lethal to cancer cells, and in mice, reduction of DHX9 expression did not have any adverse effects on body weight, blood biochemistry, and histology of various tissues compared to control mice. In data presented at the American Association of Cancer Researchers annual meeting earlier this year, Accent showed that inhibitors of DHX9 had anti-proliferative activity in MSI colorectal cancer cells with defective mismatch repair and that oral dosing of mice with the DHX9 inhibitor ATX968 resulted in robust and durable tumor regression correlating with intra-tumoral expression of the mRNA circBRIP1, a potential biomarker for clinical studies.
2023년 AACR Meeting에서 DHX9 Inhibitor 중 하나인 ATX968에 대해 발표를 했는데 mRNA circBRIP1 PD 에 Dose-Dependent Efficacy가 있슴을 보고했습니다.
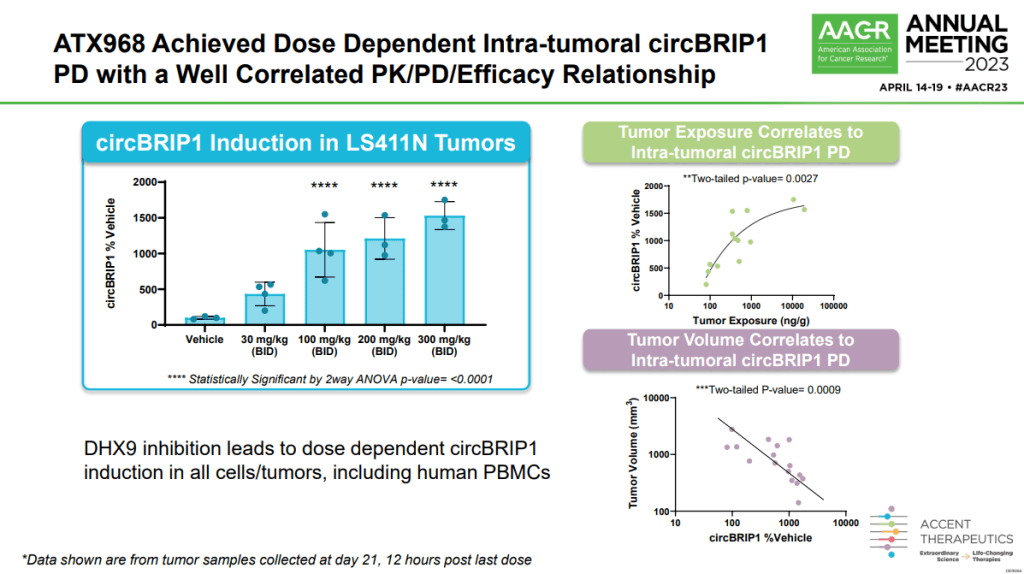
Copeland said it is likely that Accent’s initial clinical studies for DHX9 will focus on MSI-high colorectal cancer, an indication well-supported by preclinical data. However, in the course of IND-enabling studies, Accent researchers will seek to understand the full spectrum of cancers that may be treatable with a DHX9 inhibitor before committing to a plan for clinical trials.
“We’re going to make our decision on the specifics of the clinical trial design probably in the first half of 2024,” Copeland said. In considering how to position such a drug on the market, Copeland added that the company would most likely establish activity of the drug in a relapsed or refractory patient population then attempt to build a case for its use in earlier lines of therapy. But, ultimately, those expectations could change depending on data collected from clinical studies. Biomarkers for patient stratification will also be an integral part of clinical trials for a DHX9 inhibitor, Copeland noted.
In addition to its internally run drug development efforts, Accent has also been active in pursuing partnerships for its pipeline programs. In June 2020, Accent inked a deal with AstraZeneca to discover, develop, and commercialize cancer therapies targeting RNA-modifying proteins. Accent received an upfront payment of $55 million from AstraZeneca in that deal and is eligible to receive up to $1.1 billion in milestone payments, option fees, and tiered royalties based on net sales.
Thus far, Accent has not actively pursued a partnership for the DHX9 program. However, following the data presentation at AACR, Copeland said, “That talk really created a lot of interest on the part of large pharma to engage with Accent. We’ve had many different conversations, and we are open to further conversations.”
이렇듯 프로그램들의 발전에 고무된 BMS, Johnson & Johnson 등이 참여하여 Series C $75 Million을 할 수 있었습니다. DHX9과 새롭게 공개한 KIF19A 약물들을 올해 연말까지 IND Filing을 하고 2025년에 임상1상을 할 예정이라고 공개했습니다.
Two more pharmas practice their Accent, invest in $75M series C round – Fierce Biotech 1/23/2024
Accent Therapeutics has reeled in investments from two more pharmaceutical companies as part of a $75M series C, the latest validation for the RNA drug developer.
Bristol Myers Squibb and Johnson & Johnson’s investment arm, JJDC, participated in the funding round that was announced Tuesday, which was led by the recently launched Mirae Asset Capital Life Science. The two pharmas join AbbVie’s venture arm which had previously participated in the biotech’s series B.
Accent CEO Shakti Narayan, Ph.D., said the money will help drive further development of the company’s two lead small molecules, targeting DHX9 and KIF18A, respectively. Accent’s plan is to formally ask regulators to enter human trials for both programs before the end of the year and put each in phase 1 studies by early 2025.
The three pharma backers come in addition to Accent’s existing research and licensing collaboration with AstraZeneca, announced in 2020. That gave the British pharma the option to a preclinical program that Accent would develop through phase 1 studies, plus worldwide licensing rights to two additional preclinical assets should AstraZeneca want more. Narayan wouldn’t specify which programs in the pipeline, if any, were assigned to AstraZeneca, saying only that the two lead programs were wholly owned.
All told, Narayan says the attention shows that Big Pharma “is excited about our story [and] is excited about the programs we’re driving forward.”
“Together with our collaboration with AstraZeneca, there’s just a great sense of endorsement and engagement from Big Pharma around our strategy and our vision,” he said.
The DHX9 asset has the potential to be first-in-class, but the KIF18A program is lagging slightly behind Volastra Therapeutics, which has two such assets in the clinic, one of which the biotech snagged from Amgen. Naveen Krishnan, M.D., managing director of Mirae Asset Capital’s new life science fund and incoming Accent board member, said Accent could gain from Volastra’s learnings.
KIF18A Program은 Volastra Therapeutics가 Sovilnesib과 VLS-1488의 두 프로그램이 이미 임상1상을 진행 중입니다. 이 데이타를 통해 Accent Therapeutics도 향후 이 프로그램의 향방을 결정할 수 있을 것 같습니다.



“Accent had a unique approach for how they’re pursuing their KIF18 asset that to me, really focuses on widening that therapeutic index,” Krishnan said. “And I think there’s certainly room for more than one player, particularly in KIF18A.”
Accent marks the first public investment for Kirshnan and Mirae’s new life science arm, which was unveiled last week with $50 million to spend. Krishnan, who took the reins after more than two years at Leaps by Bayer, expects the fund to make up to eight investments this year. He and the firm are already finalizing plans to participate in another unnamed biotech’s financing.
오늘 현재 Accent Therapeutics의 파이프라인은 아래와 같습니다. DHX9이 가장 앞선 상태이고 KIF18A가 그 뒤를 따르고 있습니다. RNA-Modifying Proteins를 표적하는 Accent의 전략과 RNA-binding Small Molecule을 개발하는 Expansion Therapeutics 등의 전략 중 어떤 회사들이 웃을지 궁금합니다.


One thought on “BIOTECH (126) Accent Therapeutics: Small Molecules Targeting RNA-Modifying Protein”