
(Picture: Howard Federoff)
안녕하세요 보스턴 임박사입니다.
Off-the-shelf Cell Therapy는 꿈의 치료제라고 할 수 있는데 특히 Neurology 분야에서는 더욱 어렵지만 만일 할 수 있다면 환자들에게 정말 좋은 뉴스라 할 수 있습니다. 예를 들면 파킨슨씨병의 경우 Neuron의 Dopamine 분비에 이상이 생긴 것인데 이를 위한 회사 중 Autologous Stem Cell Therapy for Parkinson’s Disease인 “BlueRock Therapeutics”에 대해 블로그를 적은 적이 있습니다.
반대로 Kenai Therapeutics는 Allogeneic Stem Cell Therapy for Parkinson’s Disease”회사로서 iPSC-induced Stem Cell로 Doparminergic Neuron을 주입하는 치료법을 개발하는 것이 목표입니다. 이 회사의 CMO인 Howard Federoff 교수는 University of California at Irvine의 Vice Chancellor였기도 하고 Nasdaq 상장사인 Brooklyn ImmunoTherapeutics의 President & CEO였습니다. 하지만 그는 2022년 5월 새로운 벤처를 위해 사임합니다. 그 벤처가 바로 Kenai Therapeutics 인 것이죠.
2020년에 Howard Federoff교수는 하버드 대학교 김광수 교수의 Autologous Stem Cell Therapy for Parkinson’s Disease에 대한 New England Journal of Medicne의 논문과 관련한 글을 통해서 Autologous vs Allogeneic에 대해 평한 것이 있는데요. Redosability와 면역억제제를 복용할 필요가 없다는 점에서 Autologous Stem Cell Therapy가 보다 유용할 것으로 글을 썼습니다.
An Autologous Stem Cell-Based Therapy for Parkinson’s Disease – GEN Edge 11/25/2020 Howard Federoff
Cell therapies hold great promise to treat Parkinson’s disease, but there’s an ongoing debate over how to best do this. One group—and I am firmly in this camp—maintains an autologous therapy, derived from a patient’s own cells, is the correct path. Others believe an allogeneic approach, using donor cells, would be more cost-effective.
A recent study, published in the New England Journal of Medicine, added fuel to the fire. A research team, led by Harvard’s Kwang-Soo Kim, PhD, harvested cells from a person suffering from Parkinson’s disease, created induced pluripotent stem cells (iPSCs) and differentiated them into dopamine-producing neurons, which were ultimately transplanted into that person’s brain.
While this work generated some controversy, the procedure was well-tolerated for the study’s only participant, and that’s a first step towards establishing a safe track record for autologous neuronal cell transplants.
This narrow proof-of-concept will do little to settle the ongoing debate between allogeneic and autologous transplants. However, as the discussion moves forward, we must carefully assess all the evidence. This choice is an important inflection point for people with Parkinson’s disease—we need to make the right decisions based on the best, currently available data.
Autologous vs. allogeneic
Given the evidence, autologous therapies offer the most compelling opportunities to treat Parkinson’s disease, and give patients better quality of life, because they do not precipitate an immune response. Since allogeneic cells come from a donor and not from the person being treated, they are like heart, lung or kidney transplants: Recipients must take immunosuppressive drugs for their grafts to survive.
The jury is still out on which immunosuppressive regimens will be needed to protect allogeneic transplants from the immune system and how long those will be needed. Immunosuppression has a dramatic impact on quality of life, making people more vulnerable to opportunistic infections. The COVID-19 pandemic is an ongoing reminder that being immunosuppressed, for any amount of time, can be quite dangerous.
Since autologous transplants are generated from a person’s own cells, they will not need any immunosuppression, and that would be a big win for patients.
There’s also evidence that autologous neurons create synapses more efficiently. Presynaptic axons must scan their environments to make the appropriate connections with dendrites from other neurons. They perform this feat with such accuracy that many researchers believe axons and dendrites are encoded in some way to make those correct connections.
Alternatively, dopaminergic and other neurons can form autapses, in which they connect to themselves rather than targeting the right neurons. Though we do not fully understand how this mechanism works, we do know that neurons from autologous transplants share the same coding with their neighbors. As a result, they appear to reject autapses, favoring synapses and generating optimal connections to produce functional circuits. This may seem like a subtle detail, but it’s critically important if we want to restore function in Parkinson’s disease patients.
Another potential issue is redosing. We don’t know how long any cell transplant–autologous or allogeneic—will benefit certain patients. Parkinson patients with the sporadic (non-familial) form of the disease can start developing symptoms before they turn 50. As a result, they may need a second, or possibly even a third, transplant procedure to manage their disease.
This is an important consideration when choosing which path is the best choice for patients. If they have received an allogeneic transplant, that original cell line will have become immunogenic. However, patients who benefit from autologous transplants can continue to receive their own cells without any concerns over immunogenicity. For patients who need additional dopamine-producing neurons down the road, autologous cells are clearly the better option.
Making the fine distinctions
When assessing autologous and allogeneic transplants, we must recognize that there is significant diversity in these methods, and the scientific community should not treat them as two monolithic approaches.
For example, researchers can adopt various methods to convert mature cells into iPSCs and differentiate them into dopaminergic neurons for transplant. Kwang-Soo Kim’s group used a flavanol to destroy senescent cells during the differentiation process. This added another step that exposes transplant cells to a chemical, and that may not be the best way to proceed.
There are also different types of Parkinson’s disease, and treatments should reflect that diversity. Patients with sporadic Parkinson can receive transplanted dopaminergic neurons without any additional molecular interventions.
Patients with familial Parkinson’s disease, such as those who have mutations in their GBA gene, may be better served by autologous neuronal transplants that also deliver gene therapy. Providing patients with healthy genes, which produce appropriate amounts of the GBA enzyme, could go a long way towards restoring function.
These are just a couple examples of how complex it can be to develop cell therapies for Parkinson’s disease. This complexity should always be taken into account when assessing new therapies; apples should always be compared to apples.
Calculating cost and value
Sophisticated therapies and cost concerns often go together. On the surface, allogeneic neuron transplants would project as being less expensive than autologous therapies. Because a given cell line could serve a relatively large population of affected people, manufacturing costs could be reduced. However, there are other variables to consider.
While it’s true allogeneic lines could be scaled up effectively, there are risks associated with higher volumes. Larger scale can translate into greater risks of generating mutations in these cell lines. Some of these mutations could activate oncogenes.
Manufacturers would have to invest in elaborate quality control measures to protect patients, and that would add significant costs to the overall process.
Immunosuppression would also be costly. These drugs are expensive, and no one currently can say how long people will need them. Being vulnerable to infection generates other issues. For high-risk transplant patients, routine viral infections could lead to precautionary hospitalizations, as well as outpatient visits. For others, health-related complications from immunosuppression could lead to lengthy, expensive hospitalizations. The longer people need immunosuppression, the greater their risk.
We must also remember that cost projections to produce autologous neurons are moving targets, and they have a tendency to move lower. As we perfect iPSC protocols, and transfer those approaches from the lab to manufacturing, we will continue to automate processes and identify other efficiencies. By implementing the most innovative manufacturing protocols, companies can significantly reduce the cost of producing autologous neurons.
If we factor in all these expenses, the cost advantages associated with allogeneic transplants tend to dissipate. Still, costs are not exclusively a monetary realm. Some have expressed concerns that producing neurons for autologous transplant will take too long, and patients may decline precipitously while waiting.
This is actually a red herring. Mature cells can be converted into iPSCs and subsequently differentiated into dopaminergic neurons in less than four months. During that short period, it’s unlikely that a Parkinson patient eligible for this type of therapy would experience any clinically-detectable deterioration.
We still have a long way to go with cell therapies. To move forward with autologous neuron transplants, we need to approach them in a rigorous and systematic way, conduct robust, well-designed clinical trials to study safety, tolerability and efficacy and develop innovative neurosurgical approaches.
그러나, 그의 연구는 Augologous가 아닌 Allogeneic iPSC-derived dopamine progenitor cell Therapy였는데 2022년 NPJ Regenerative Medicine에 연구결과를 발표합니다.
Brooklyn ImmunoTherapeutics CEO Howard Federoff leaves – Exchange 5/31/2022
This news came shortly after Memorial Day. Howard Federoff, chief executive of Brooklyn ImmunoTherapeutics, leaves. As announced by Brooklyn ImmunoTherapeutics Inc. in a news release and in a regulatory filing published on Tuesday, May 31, 2022, Howard J. Federoff has left his post as chief executive officer at the biopharmaceutical company, after about a year in the role, effective May 26, 2022.
Howard Federoff’s duties as CEO will be taken over temporarily by Matthew (Matt) Angel, most recently Chief Executive Officer at Factor Bioscience Inc., as Interim Chief Executive Officer.
The management change is explained as follows. Brooklyn ImmunoTherapeutics said: “Brooklyn ImmunoTherapeutics, Inc. (Nasdaq:BTX) (“Brooklyn” or the “Company”), a biopharmaceutical company focused on exploring the role that cytokine, gene editing, and cell therapy can have on the immune system for treating patients with cancer, blood disorders, and monogenic diseases, today announced the appointment of Matt Angel, Ph.D., Co-Founder, Chairman, and CEO of Factor Bioscience Inc., as Interim Chief Executive Officer and President. He will replace Howard J. Federoff, M.D., Ph.D., Chief Executive Officer and President, who departs to focus on building a new venture.”
Precise information regarding Howard Federoff’s future plans was not immediately available.
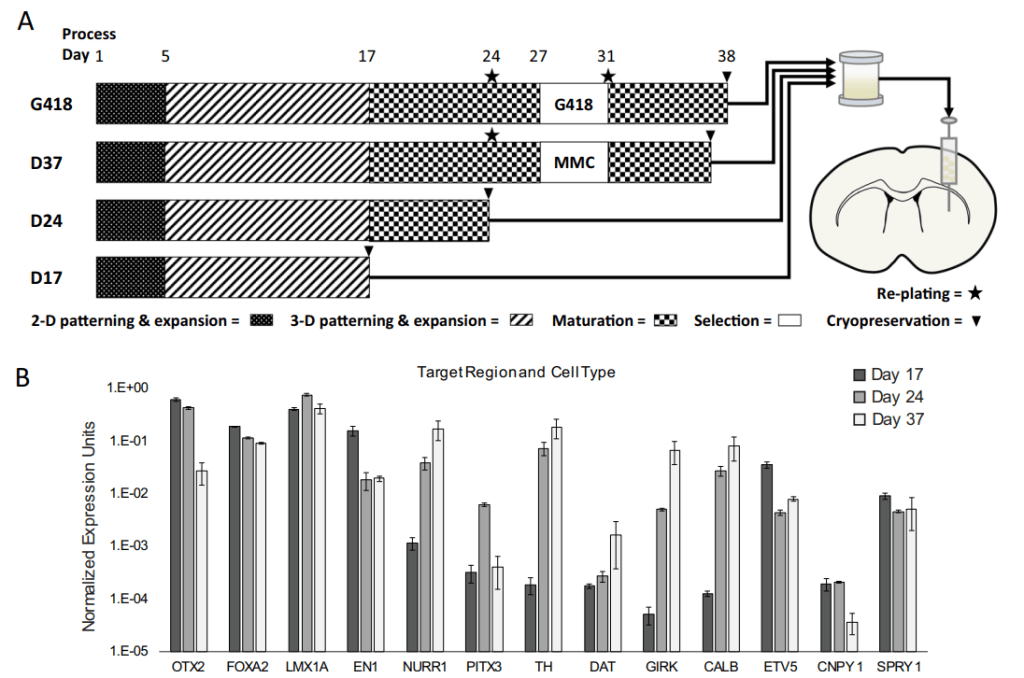
Kenai Therapeutics (previously Ryne Biotechnology)는 CIRM으로 부터 $4 Million grant를 받아서 RNDP-001이라는 iPSC-derived dopamine neuron progenitor 약물의 전임상을 할 수 있는 초기 자금을 확보합니다.
Ryne Biotechnology, Inc. (Ryne Bio), a therapeutics company leveraging induced pluripotent stem cell (iPSC) technology to discover and develop a platform of off-the-shelf neuron replacement therapies for neurological disorders, today announced that the California Institute for Regenerative Medicine (CIRM) has awarded the company a $4 million Clinical Stage Research Program (CLIN1) grant. This funding will enable the company to advance its lead candidate RNDP-001, an iPSC-derived dopamine neuron progenitor for the treatment of both inherited and idiopathic forms of Parkinson’s disease, through submission of an Investigational New Drug (IND) application within the next 12 months.
RNDP-001 has completed preclinical efficacy and safety studies. This CIRM award will allow Ryne Bio to finalize its IND-package including the production of GMP-grade materials to enable the evaluation of RNDP-001 in Phase 1 clinical trials for both inherited and idiopathic forms of Parkinson’s disease.
“We appreciate CIRM’s partnership in our vision to reverse degenerative conditions of the brain by developing off-the-shelf cell replacement therapies,” said Nick Manusos, Chief Executive Officer of Ryne Bio. “A dramatic shift in the standard of care for patients with neurodegenerative disease is long overdue. We are thrilled to be developing groundbreaking therapies for patients in need of better treatment options.”
Beyond RNDP-001, Ryne Bio is developing a platform of drug candidates, including next-generation, gene-modified programs that have the potential to modify and reverse disease progression in Parkinson’s disease and other moderate to severe central nervous system disorders.
“The underlying cause of Parkinson’s disease is progressive degeneration of a patient’s dopamine neurons. Ryne Bio is able to directly replace dopamine neurons that have been lost by utilizing precision manufacturing techniques,” said Howard Federoff, M.D., Ph.D., Ryne Bio’s Chief Medical Officer, scientific co-founder and Principal Investigator on the CLIN1 award.
In addition to funding from CIRM, Ryne Bio was launched and seeded in 2022 by Saisei Ventures, an emerging venture capital firm focused on building revolutionary advanced medicine companies. “The potential of off-the-shelf cell replacement therapies is on the cusp of being realized for complex and intractable disease,” said Jonathan Yeh, Managing Partner of Saisei Ventures. “This funding decision from CIRM provides robust validation of the Ryne Bio approach, and supports the delivery of this best-in-class therapy to patients.”
그리고 1년 후 $82 Million Series A를 통해서 RNDP-001의 임상 준비에 박차를 가할 수 있는 자금을 확보하게 됩니다. 보스턴에 있는 Fujifilm Cellular Dynamics에서 연구와 GMP 생산을 하기로 계약이 되어 있고 RNDP-001을 IND filing하고 임상에 진입함과 동시에 다른 Neurological Diseases에 대해서도 파이프라인을 늘려 간다는 계획입니다. BlueRock의 Autologous Stem Cell Therapy와 함께 Kenai의 Allogeneic Stem Cell Therapy가 Parkinson’s Disease 환자들에게 희망이 되어줄 수 있을지 기대가 됩니다.
Kenai Emerges from Stealth With $82M Series A, Targets Parkinson’s – Biospace 3/1/2024
San Diego-based Kenai Therapeutics announced its arrival on the biotech scene Thursday with $82 million in Series A financing to fund an investigational asset designed to treat Parkinson’s disease.
According to Kenai, which was formerly known as Ryne Bio, the funding was co-led by Alaska Permanent Fund Corporation, The Column Group and Cure Ventures. The round also saw participation from Saisei Venture and Euclidean Capital.
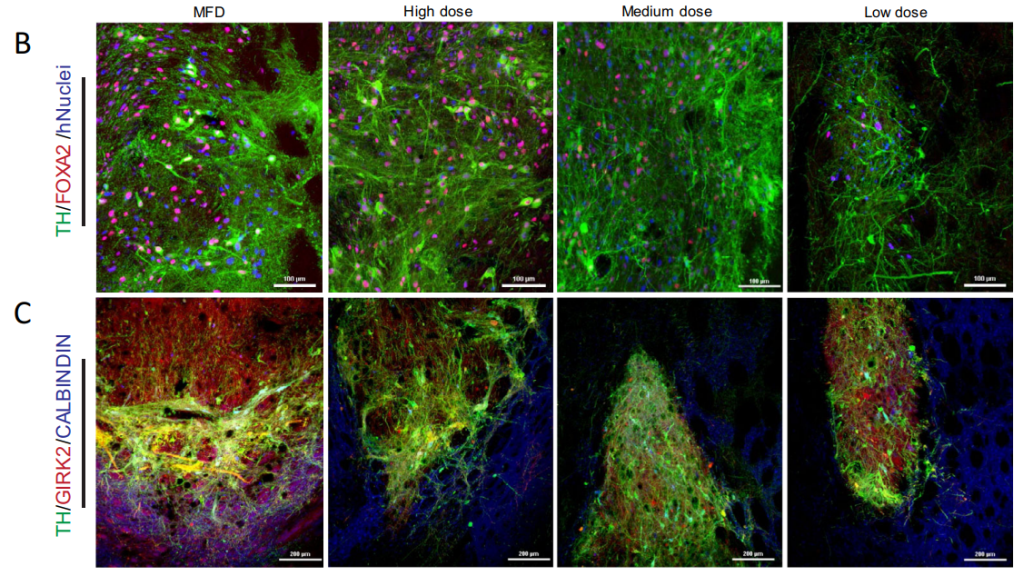
The Series A is meant to help Kenai submit an IND for RNDP-001, an iPSC-derived allogenic dopamine progenitor cell therapy intended to treat idiopathic and inherited forms of Parkinson’s. Funding will also go toward the completion of Phase I trials which will start sometime this year.
Kenai said the asset has shown “robust survival, innervation, and behavioral rescue” in preclinical models.
“We are grateful for the support of a syndicate of leading life science investors and a team of industry veterans, including scientific co-founders Dr. Howard Federoff and Dr. Jeffrey Kordower, who see the promise in Kenai’s approach to treating central nervous system disorders,” Kenai CEO Nick Manusos said in a statement. “Their guidance will be invaluable as we soon advance our lead candidate, RNDP-001, into the clinic to treat Parkinson’s disease.”
In addition to the Parkinson’s candidate, Kenai is pursuing a pipeline of off-the-shelf dopamine neuron replacement cell therapy assets targeting neurological disorders. However, no other specific disease targets were revealed in the announcement. Kenai is using Fujifilm Cellular Dynamics for its manufacturing and development services.
“Kenai’s proprietary platform leverages an emerging approach to treating central nervous system disorders by replacing neurons lost due to neurodegeneration,” Jeff Jonas, chair and board member of Kenai Therapeutics and partner at Cure Ventures, said in a statement. “The potentially curative nature of RNDP-001 for Parkinson’s disease could dramatically alter outcomes for patients with very few treatment options.”
In February 2023, when the company was known as Ryne Bio, it received a $4 million grant from the California Institute for Regenerative Medicine to support the development of RNDP-001.
