This Startup Is Rewriting The Language Of Life To Make Smart Biologics – Forbes 6/20/2023
Synthetic biology has brought many breakthroughs to the biotherapeutics space over the last decade. The dropping cost of sequencing and precision genome editing has paved the way for personalized medicine. At the same time, generative AI technology has enabled the designing of antibodies with a much higher clinical success rate. Yet, scientists’ ingenuity is still challenged by the laws of biology. All biologics are susceptible to unpredictable degradation rates and immune responses, in addition to being constrained to the 20 natural amino acids that make up these therapeutics.
But now, one company is challenging that paradigm. Pearl Bio, a synthetic biology company backed by Khosla Ventures, has recently emerged from stealth mode with a hefty IP portfolio of 24 patents that protect their groundbreaking technology: a genetically recoded organism. With it, Pearl Bio is creating entirely new classes of materials for smart biologics.
Pearl’s genetically recoded organism, combined with the engineered translational machinery of the cell, breaks the rules of life by enabling the incorporation of building blocks beyond the 20 amino acids that exist in nature. This technology can disrupt the medical paradigm by producing novel chemistries that solve existing challenges in the immunotherapeutics space and pave the way to completely new materials.
What is a Genetically Recoded Organism (GRO)?
Pearl’s cornerstone technology is a genetically recoded organism (GRO), which heralds a new era of synthetic biology. The rules of life are written in a four-letter genetic code: A, T, C, and G. These letters form three-letter words—called codons—that encode the amino acids which make up the tissues and enzymes of all living beings. However, biology is not perfect: there are 64 possible combinations of ATCG but only 20 amino acids. This phenomenon is known as “redundancy.”
The idea of using those redundant codons for a practical purpose has been around for a while. “It dates back to the late 2000s. I was working as a postdoc in George Church’s lab, together with Michael Jewett,” recalls Farren Isaacs, Co-founder and Science Advisor at Pearl Bio. “We were always the first people to get to the lab in the morning. There was no one around at that time, and we would brainstorm ideas.”
Isaacs and Jewett, Pearl Bio’s other Co-founder and Science Advisor, were working on the bleeding edge of synthetic biology at that time: “I was recoding genomes, and Michael was engineering ribosomes. We realized that when you put those two technologies together, you have the ability to basically repurpose the genetic code and the translational machinery of any cell to produce entirely new materials,” says Isaacs.
In 2013, scientists from the Isaacs lab managed to free up one of those three-letter combinations by editing the entire E. coli genome. Now the missing codon could be assigned to code for anything else, such as non-natural amino acids, which do not occur in any living beings. By introducing these new-to-nature building blocks, you could make proteins that are impervious to degradation, target specific tissues and disease states, and attach highly specialized payloads.
“That could lead to fundamentally new kinds of therapeutics that have longer stability and reduced immunogenicity,” says Isaacs.
A New Way to GRO
Years went by, but Isaacs, Church, and Jewett kept working on tweaking the technology to improve and expand on what it could do. In 2020, the two decided to co-founder Pearl Bio together with Amy Cayne Schwartz, Chief Operating Officer & Chief Business Officer, and George Church and Jesse Rinehart of Yale as Science Advisors. Pearl Bio was officially financed and launched in Q3 of 2021.
“This is something that we’ve been incubating over many years: developing the technology, de-risking proof of concept, and filing IP,” says Isaacs. “With platform technology companies, you want to be poised to advance product development from day one.”
And Pearl Bio was. They had been working to secure an impressive portfolio of 19 patents to corner the market of multi-functionalized biologics. The newly issued U.S. patent 11,649,446 gives Pearl Bio the exclusive license for engineering programmable biologics by encoding synthetic chemistries and now brings their total patent figures to 24.
When the paper describing the first GRO was published in 2013, the technology and the strain were released publicly. But just like the first version of iOS, there were many things that needed to be improved to make the strain better suited for commercial applications. For example, the initially published version only had one codon freed up, which meant that it could only encode one alternative amino acid. Pearl took this concept further and now also holds exclusive rights to a newly developed GRO with two open coding channels to endow multi-functionality into protein therapeutics.
“Another key piece of IP is the orthogonal tethered ribosomes. This allows Pearl to engineer the ribosome to work efficiently with exotic substrates beyond L-alpha amino acids, opening access to new classes of therapeutic biomaterials. This capability holds promise to transform biopharmaceutical development,” says Jewett.
Other companies have also taken a stab at challenging the paradigm within the biotherapeutics space. For example, Ambrx, a clinical-stage biopharmaceutical company using an expanded genetic code technology, IPO’d for $126 million in 2021. However, Ambrx’s technology does not use genetically recoded organisms. Synthorx is another synthetic genome company in this space which was acquired by Sanofi for $2.5 billion in 2019. Their Expanded Genetic Alphabet technology that adds a novel DNA base pair can be used to create optimized biologics. GRO Biosciences, a company in Cambridge, MA, is expanding the amino acid alphabet to deliver on the promise of protein-based therapies. Their platform comprises Genomically Recoded Organisms (GROs) for high-efficiency production of non-standard amino acid (NSAA) proteins at commercial scale.
What distinguishes Pearl Bio is that they hold a number of broad-blocking patents giving Pearl the exclusive right to encode synthetic amino acids using engineered synthetases and translation machinery to drive multi-site incorporation of synthetic amino acids and other building blocks with site-specificity at high yield and purity.
Schwartz thinks this technology is poised to overcome a lot of the shortcomings of current biologics on the market: “For example, optimizing the drug-antibody ratio has been a defining challenge because when you attach the drug to natural amino acid residues, you are limited in the number and specific location since multiple lysines, for example, are found in a given protein and are critical for its function. Thus, approaches that attach to natural amino acids are constrained, lead to heterogeneous products, and typically ablate protein function. By contrast, Pearl can attach up to 50 synthetic amino acids at monomeric precision to tune therapeutic properties while preserving protein sequence and function.”
Such precision enables the programmability of a therapeutic window with a half-life of up to three weeks to address specific disease indications or patient populations. “With this, we have the opportunity to advance both best-in-class and first-in-class therapeutics to address critical unmet needs and solve challenges plaguing the biologics industry,” says Schwartz.
The Future of Biotherapeutics
Pearl has positioned itself as the leader in this new field thanks to advancing technology and staking the IP landscape. They have developed exclusive next-generation multifunctional capabilities, such as adding synthetic monomers, multiple types of modifications, and multiple locations.
“It’s exciting to see ideas from whiteboards over 15 years ago realized in technologies that are now poised to transform the therapeutic landscape and evolve novel biomaterials. Pearl is pioneering a new era of biotherapeutic design by enabling access to new disease targets and evolution of entirely new classes of molecules,” says Church.
“Today, we can access two distinct functionalities. With our Series A funds, we will advance the technology to access three or more distinct functionalities,” says Schwartz. “We are excited to drive next-generation molecules to the clinic to change the therapeutic landscape with the evolution of smart programmable biologics.”
Pearl Bio Secures Breakthrough IP for Multi-Functionalized Biologics – Business Newswire 6/20/2023
Pearl Bio, a synthetic biology company backed by Khosla Ventures, is recoding life to create a new era of biologics and biomaterials. A newly issued breakthrough U.S. patent 11,649,446 related to engineering programmable biologics by encoding synthetic chemistries bolsters Pearl’s patent portfolio to corner the market of multi-functionalized biologics with Pearl’s exclusive license to the issued patent. The Pearl team includes world leaders in synthetic biology, genome recoding, and ribosome engineering: Drs. George Church (Pearl Bio, Scientific Advisory Board), Farren Isaacs (Yale University), Michael Jewett (Stanford University) and Jesse Rinehart (Yale University).
“By encoding diverse synthetic chemistries into proteins, Pearl is able to tune half-life, target delivery to diseased cells, and attach cytotoxic payloads to tailor valuable therapeutic properties, overcoming key barriers preventing market approval,” explained Co-Founder Amy Cayne Schwartz. Pearl’s platform leverages 24 exclusively licensed patents and applications evolved over the last decade by the company’s scientific Co-Founders, Dr. Isaacs and Dr. Jewett. The company is advancing partnerships with pharmaceutical companies alongside internal programs to develop next-generation “smart” biologics.
Bringing together the newly-issued patent with existing broad blocking patents on genomically recoded organisms, tethered ribosomes and engineered translational machinery enables access to new frontiers by site-specifically encoding synthetic monomers to derive novel biologics and biomaterials.
Pearl’s technology preserves the natural protein activity while endowing valuable therapeutic properties to address defining challenges in biologic drug development – toxicity, stability, and targeted delivery – fast-tracking the path to market. For example, compounds designed to sustain presence of a cytotoxic payload in the tumor microenvironment coupled with access to novel targets will open-up entirely new therapeutic opportunities to address unmet medical needs and transform patient quality of life.
About Pearl Bio
Backed by Khosla Ventures, Pearl Bio was launched by Scientific Co-Founders Drs. Farren Isaacs (Yale), Michael Jewett (Stanford), and Amy Cayne Schwartz, J.D. (Pearl Bio) bringing together 24 patents in a platform technology to advance multi-functionalized biologics and biomaterials by encoding synthetic chemistries. Broad blocking patents afford freedom to operate, and the company has rapidly advanced capabilities in-house and through pharmaceutical partnerships. Pearl Bio may be followed at: pearlbio.com Twitter: https://twitter.com/PearlBio
Patent Issued for Synthetic Peptide Chain Techniques – Science & Enterprise 6/20/2023
20 June 2023. A new U.S. patent, licensed to a start-up biotechnology company, describes processes for linking together chains of synthetic peptides into therapeutic proteins. The U.S. Patent and Trademark Office issued patent number 11,649,446 last month to four inventors at Yale University and elsewhere, including a founder of the company Pearl Bio in Cambridge, Mass. that acquired rights to the technology.
Pearl Bio is a two year-old enterprise discovering synthetic combinations of peptides, short chains of amino acids, for new therapies and bio-based materials. The company is commercializing research by biomedical engineering labs of its scientific founders Farren Isaacs at Yale University and Michael Jewett at Stanford University, as well as Yale physiology professor Jesse Rinehart and Harvard Medical School geneticist George Church. Pearl Bio says the new patent is the latest of 24 patents it licenses exclusively for its basic technology.
The Farren Isaacs lab at Yale studies genome engineering techniques, particularly for high-volume programming of genetic chemistries in single cells and across cell populations. The lab says its discoveries make possible large-scale assembly of genomes into hierarchies that express genetic modifications to achieve desired outcomes, even new organisms if needed. This capability includes engineered protein synthesis in the ribosome, where messenger RNA translates and sequences genetic codes into chains of amino acids to form peptides, then linking together peptides into longer multiple peptide chains and proteins.
Produce synthetic proteins in greater yields
The new patent, which lists Isaacs as the lead inventor and assigned to Yale University, describes processes for preparing multiple peptide chains from amino acids, particularly amino acids not normally found linked together in their natural states. The patent includes methods for producing synthetic proteins in greater yields than conventional processes, or where conventional techniques would not allow for those combinations of amino acids to produce adequate yields or purity, or even occur in some cases.
“By encoding diverse synthetic chemistries into proteins,” says Pearl Bio co-founder and chief operating officer Amy Cayne Schwartz in a company statement released through BusinessWire, “Pearl is able to tune half-life, target delivery to diseased cells, and attach cytotoxic payloads to tailor valuable therapeutic properties, overcoming key barriers preventing market approval.”
Pearl Bio says its synthetic proteins retain their natural protein activity, but still allow for tuning the protein chemistry to reach specific targets, maintain stability, and reduce toxicity. The company cites as examples delivery of cancer-killing proteins to tumors through the protective microenvironment, while also accessing novel therapeutic targets. Pearl Bio says it’s forming partnerships with drug makers to develop what the company calls the next generation of smart biologics. In addition, says the company, its process makes possible genetically altered organisms that can produce strings of basic components constructed into wholly new biologics and bio-based materials.
Merck & Co. has signed up to Pearl Bio’s synthetic biology platform, offering up to $1 billion in biobucks for potentially cancer-busting biologics.
The collaboration could pile more biologics onto Merck’s pipeline and serves as validation for Pearl, which emerged from stealth in June 2023 with the backing of Khosla Ventures. Merck is paying an undisclosed upfront fee and offering up to $1 billion in milestone payments for Pearl to identify biologics to treat cancer.
The biotech describes itself as creating “template-directed biomaterials with tunable properties.” Co-founder, Chief Operating Officer and Chief Business Officer Amy Cayne Schwartz elaborated in an email to Fierce Biotech, describing how the platform can “encode synthetic chemistries to tune therapeutic properties (eg half-life, drug antibody ratio.” The company is in the process of raising its series A round, she told Fierce.
“Pearl Bio is recoding life for a new era of programmable or ‘smart’ biologics,” Schwartz wrote. “Series A resources will be used over the next two years to drive molecules to the clinic and further differentiate our technology with the ability to endow three distinct functionalities with tunable properties into biologics.”
Pearl was spun out of research from Farren Isaacs, Ph.D., and Michael Jewett, Ph.D., that centers on using ”genomically recoded organisms” to add synthetic chemistry to biologics. The company counts famed molecular engineer and serial entrepreneur George Church, Ph.D., as one of its scientific advisers. In June 2023, the U.S. granted a patent to Pearl and its founders that bolsters their multifunctional biologic ambitions, cementing future use of tools like synthetic amino acids and tethered ribosomes.
As for Merck, it’s just the latest collab to land on its conveyor belt of licensing pacts. The Big Pharma’s recent slate of bets has largely focused on oncology, including deals with C4 Therapeutics and Daichii Sankyo, alongside the $680 million acquisition of Harpoon Therapeutics.
Juan Alvarez, Merck’s vice president of discovery biologics at Merck Research Labs, described Pearl in a release this morning as a “pioneer in developing recoded organisms.”
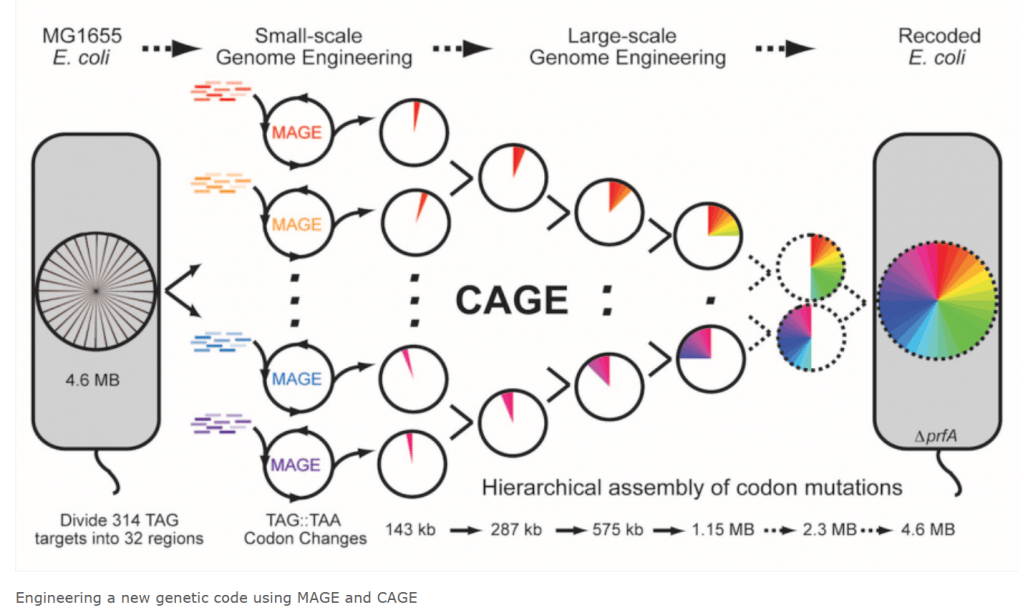
Farren Issacs Lab at Yale University 에 보여진 연구내용을 보면 MAGE (Multiplex Automated Genome Engineering)과 CAGE (Conjugative Assembly Genome Engineering) 을 통해서 Recorded E. Coli 를 만들 수 있습니다. Hierarchical assembly of codon mutations를 통해 다양한 사이트에 정확도 높은 조작을 할 수 있다는 설명입니다.