안녕하세요 보스턴 임박사입니다.
Opioid 약물을 FDA에서 승인해 준 이래로 Opioid 약물로 인한 여러가지 부작용으로 인한 Opioid Endemic이 연일 기사를 도배하고 있습니다. 이런 가운데 Vertex는 최근 Non-Opioid Replacement NaV1.8 Inhibitor Suzetrigine에 대한 기대되는 임상3상 결과를 발표했습니다.
BIOTECH (92) – Vertex Non-Opioid Replacement NaV1.8 Inhibitor Suzetrigine (VX-548) Phase 3
Amgen 출신의 Sean Harper 박사 등이 세운 VC 인 Westlake Village BioPartners가 2020년에 Suzetrigine에 대항하는 Best-in-class NaV1.8 Inhibitors를 개발하기 위해 Amgen 연구원들을 모아서 Latigo Biotherapeutics를 설립했습니다.
BIOTECH (82) – Westlake Village BioPartners VC
Amgen에서 NaV1.7 Inhibitor 개발을 했던 Bryan Moyer박사가 Co-founder and SVP Discovery로 참여하고 있습니다.

Latigo Biotherapeutics의 홈페이지에 나온 파이프라인을 보면 LTG-001이 임상1상을 진행 중이고 Follow-on molecule (NaV1.8 Inhibitor) 가 전임상 단계에 있습니다.
Latigo Biotherapeutics는 Amgen Neuroscience와 연관이 있습니다. 기사를 통해서 보면 Amgen은 2015년에 Novartis와 AMG 334 (Erenumab)라는 Calcitonin-Gene-Related Peptide (CGRP) receptor를 공동개발한다고 발표한 바 있습니다. 이 당시 Novartis의 BACE Inhibitor도 공동개발에 함께 들어가 있었습니다.
Amgen, Novartis Launch Neuroscience Drug Collaboration – GEN Edge 9/2/2015
As for the migraine program, the companies said they will work to co-develop new Amgen drugs that include the Phase III compound AMG 334, the Phase I compound AMG 301, and potentially another Amgen compound. Novartis will have global co-development rights and commercial rights to Amgen’s migraine treatments outside the U.S., Canada, and Japan.
AMG 334 is a fully human monoclonal antibody under study for the prevention of migraine. AMG 334 targets the Calcitonin-Gene-Related-Peptide (CGRP) receptor, which according to Amgen is believed to transmit signals that can cause incapacitating pain. AMG 334 is currently under evaluation in several large global, randomized, double-blind, placebo-controlled trials to assess its safety and efficacy in migraine prevention. AMG 301 is a monoclonal antibody being investigated for the treatment of migraine.
“Our collaboration on BACE inhibition reflects Amgen’s strategic focus on genetically validated drug candidates while our collaboration in migraine creates an opportunity to more rapidly advance AMG 334 on a global scale,” Sean E. Harper, M.D., Amgen evp of research and development, said in a statement.
이 약물 Aimovig (AMG 334, Erenumab)은 2018년에 FDA로 부터 승인을 받았습니다.
FDA approves novel preventive treatment for migraine – FDA Press Release 5/17/2018
The U.S. Food and Drug Administration today approved Aimovig (erenumab-aooe) for the preventive treatment of migraine in adults. The treatment is given by once-monthly self-injections. Aimovig is the first FDA-approved preventive migraine treatment in a new class of drugs that work by blocking the activity of calcitonin gene-related peptide, a molecule that is involved in migraine attacks. The FDA granted the approval of Aimovig to Amgen Inc.
Pain Medicine으로 Amgen에서는 오랜 기간 NaV1.7 Inhibitor의 개발에 투자를 해 왔습니다. Latigo의 SVP Discovery인 Bryan Moyer박사는 2017년에 Journal of Medicinal Chemistry에 NaV1.7 Inhibitor의 Lead Optimization에 관한 논문을 발표한 바 있습니다.
그리고 같은 해에 Bryan Moyer박사 등은 2017년에 Journal of Pharmacology and Experimental Therapeutics에 NaV1.7 Inhibitor인 AMG8379에 대해 발표한 바가 있습니다.

뿐만 아니라 2017년에는 Boston Children’s Hospital과 새로운 Pain Syndrome Targets를 발굴하기 위한 Genetic collaboraton을 발표한 바 있습니다. 이렇듯 Amgen의 Neuroscience 부문에서 Pain과 관련한 연구는 오랜기간 지속적으로 발전해 왔습니다.
Amgen (NASDAQ:AMGN) and Boston Children’s Hospital today announced that they have entered into a neuroscience research collaboration aimed at identifying novel pain targets based on human genetic analyses. The one-year collaboration will focus on patients with genetic anomalies of pain sensitivity. Amgen will leverage its industry-leading expertise in genetic target identification and validation and will have access to Boston Children’s Hospital’s Division of Pain Medicine to identify patients with abnormal pain conditions. Amgen and Boston Children’s Hospital will collaborate to validate the genetic findings as potential pain targets.
“Traditional approaches to analgesic drug discovery have been pretty disappointing during the past 20 years,” says Charles Berde, M.D., Ph.D., chief of the Division of Pain Medicine in the Department of Anesthesiology, Perioperative and Pain Medicine at Boston Children’s Hospital. “The most innovative biotech companies have realized that they need to pursue new directions for drug discovery. Patients with unusual patterns of increased or decreased pain responsiveness can offer important clues in this pursuit.”
“Amgen is pleased to enter into this collaboration as it underscores our extensive investment and expertise in pursuing targets that have clear genetic support,” said John Dunlop, Ph.D., vice president of Neuroscience Research at Amgen. “We look forward to working with Boston Children’s Hospital to explore novel pain targets that will potentially include new non-addictive approaches to treating pain in patients.”
The agreement brings Amgen, a world leader in human genetic target validation, together with Boston Children’s Hospital’s Division of Pain Medicine, the first and most active pediatric pain program in the world, and its Manton Center for Orphan Disease Research. Both organizations have leading researchers in neuroscience and genomics, including Michael Costigan, Ph.D., of the F.M. Kirby Neurobiology Center and Catherine Brownstein, M.P.H., Ph.D., in the Division of Genetics and Genomics and scientific director of the Manton Center for Orphan Disease Research.
Boston Children’s Hospital’s Division of Pain Medicine treats patients with rare conditions that make them strikingly insensitive to pain or, conversely, hypersensitive to pain or apt to experience pain spontaneously, with no apparent stimulus.
As part of the collaboration, the teams will study patients with the following pain syndromes:
- genetic disorders that diminish pain sensitivity;
- erythromelalgia, a condition causing intense, burning pain in the extremities;
- paroxysmal extreme pain disorder, a condition characterized by skin flushing and severe pain attacks in various parts of the body; and
- hereditary sensory and autonomic neuropathy.
그러나 2019년 10월말에 Amgen은 Neuroscience R&D 부문을포기하고 Layoff를 하게 됩니다. 항상 위기는 누군가에게 기회가 됩니다. Amgen Neuroscience EVP였던 Sean Harper 박사는 Westlake에서 새로운 벤처 스타트업을 생각하고 있었는데 Amgen이 Novartis와 공동으로 개발하던 BACE Program이 Alzheimer치료제로서 효과를 보이지 않자 뇌과학 분야를 중단한다는 발표는 Sean Harper 박사에게는 기회가 되었습니다. 2020년에 Bryan Moyer 박사를 비롯한 ex-Amgen Neurosciences Drug Hunters들을 모아 Latigo Biotherapeutics를 설립할 수 있었습니다.
Amgen exits neuroscience R&D as pharma pulls back from field – Biopharmadive 10/30/2019
Recent scientific breakthroughs have transformed treatment of some cancers, as well as a number of rare genetic diseases. But disease-modifying medicines for the most prominent diseases of the brain, such as Alzheimer’s and Parkinson’s, have remained elusive.
Earlier this year, Amgen reported a major clinical setback in Alzheimer’s. Along with its partner Novartis, the drugmaker stopped two studies testing an experimental BACE inhibitor in pre-symptomatic patients with the neurodegenerative disease.
The trial failure was one of many for the Alzheimer’s field, but, for Amgen, it brought to a close the only named clinical neuroscience program in its pipeline outside of the approved migraine therapy Aimovig (erenumab).
Now, Amgen will steer its internal R&D efforts clear of neuroscience, prioritizing instead cardiovascular disease, oncology and inflammatory diseases. As a result of the decision, approximately 180 roles will be affected, according to a company spokesperson.
“We made the difficult decision to end our research in neuroscience, which is largely based in Cambridge, Mass.,” said the spokesperson in an emailed statement. “We are consolidating our U.S.-based research presence primarily in Thousand Oaks and San Francisco.”
On Tuesday’s earnings call, company executives previewed how Amgen could stay involved via partnerships. CEO Bob Bradway said they’ll explore models with venture capital or academic institutions, particularly via deCODE, a subsidiary specializing in genetics, to better understand these diseases.
“We believe that genetics will ultimately drive progress in this area, and we’ll continue to work with deCODE to generate insights,” Bradway said on the Tuesday call.
Reese also added the company will maintain support for the ongoing clinical development of its migraine therapy, Aimovig (erenumab).
Amgen’s decision bears similarities with those taken by some of its peers.
Pfizer, for instance, first announced its intention to halt neuroscience work in January 2018. Several months later, the big pharma teamed up with Bain Capital to launch a start-up called Cerevel that took over development efforts for many of Pfizer’s CNS compounds.
Still, there are a handful of industry players that have bucked the trend. Last month, the Danish drugmaker Lundbeck reached a deal to acquire Alder BioPharmaceuticals for $1.95 billion.
And Biogen has remained focused on developing central nervous system treatments, even as it has suffered multiple clinical failures. In a shocking turn, the big biotech recently revived development efforts for an experimental Alzheimer’s drug.
Latigo Biotherapeutics는 Johns Hopkins University의 Lieber Institute와 새로운 NaV1.9 Inhibitor에 대해 2022년 10월에 발표한 적이 있습니다. 이 약물의 적용분야로 거론된 것은 진통제, 관절염, 심장병, 위경련, 암, 정신병 등 광범위한 분야의 통증완화 치료제의 개발이었습니다.
Latigo Biotherapeutics, Lieber Institute present new Nav1.8 channel blockers – BioWorld 10/6/2022
Latigo Biotherapeutics Inc. and Lieber Institute Inc. have synthesized new methyl-substituted pyridine and pyridazine compounds acting as sodium channel protein type 10 subunit α (Nav1.8) channel blockers reported to be useful for the treatment of pain, arthritis, atherosclerosis, irritable bowel syndrome, cancer and psychiatric, respiratory and neurological disorders, among other disorders.
Latigo 의 특허 WO 2022/192487 A2에 따르면 Lieber Institute of Brain Research at Johns Hopkins University 의 Dr. James Barrow와 Dr. Michael Poslusney 라는 Merck 출신 과학자들이 LTG-001의 개발에 참여했습니다.

2022년에 발표한 Lieber Institue의 발표에 의하면 4,000개 이상의 뇌질환 환자 시료를 보유하고 있고 NeuroGenetics 연구에 강점을 가진 연구소임을 알 수 있습니다.
그리고 지난달에 $135 Million Series A를 했다는 발표를 했습니다. 이미 LTG-001이 임상1상에 진입을 한 상태인데 Vertex를 속히 따라잡기 위해서 조기에 임상1상을 완료하고 다양한 임상2상을 시작한다는 계획입니다. Vertex 약물이 primary endponts는 좋게 나왔지만 secondary endpoint는 아직 확실한 승리를 장담할 수 없다고 생각하고 이런 Vertex 약물 Suzetrigine (VX-548)의 약점을 파고든다는 전략입니다. 임상2상에서는 Chronic Pain 에 대해서도 임상시험을 계획하고 있습니다.
A new pain-focused biotech is emerging this Valentine’s Day—not to cure heartache, but pretty close. Latigo broke stealth cover today with $135 million to chase Vertex, the front runner in the effort to develop a new non-opioid pain option.
Latigo Biotherapeutics unveiled Wednesday with a lead nav1.8 inhibitor, LTG-001, currently being tested in healthy volunteers. If that target sounds familiar, it’s because Vertex is going after it as well with its own non-opioid treatment. VX-548 just beat placebo in a phase 3 trial in patients with acute pain but did not manage to best the standard of care, Vicodin. Vertex plans to file for FDA approval anyway this year and is seeking a broad label in moderate to severe acute pain.
Sean Harper, M.D., co-founding managing director of Westlake Village BioPartners, which founded and incubated Latigo beginning in 2020, said beating opioids is a “tough bar.”
“I think a goal in an acute pain setting to beat that type of comparator handsomely … would have to be [an] aspiration,” he said in an interview. With that said, Harper believes Latigo’s molecule will differentiate from Vertex’s by mitigating off-target effects in the brain and by tackling chronic pain.
“We have every reason to believe that our nav1.8 inhibitor will be effective in both acute and chronic pain,” Harper said in an interview. “But obviously, that remains to be proven.”
The asset is in a healthy volunteers study now, and executives hope to initiate a phase 2 study for patients with acute pain later this year.
Harper said some of the foundational science behind LTG-001 originated from the Lieber Institute for Brain Development in Baltimore but that preclinical work was done at labs in Thousand Oaks, California. The VC firm plucked homegrown talent for help, hiring many Amgen employees who left as part of the pharma’s neuroscience divestment in late 2019. The next molecule behind LTG-001 was developed completely in-house, Harper said, in addition to other discovery work.
Desmond Padhi, an operating partner at Westlake, will be the interim CEO while Neuron23 CEO Nancy Stagliano, Ph.D., has been called upon to chair the board. Although corralling investor interest is never easy, Stagliano acknowledged that Latigo has “hit this important catalyst at just the right time.”
“Once in a while, you get the timing to be perfect,” she said.
The inaugural financing was co-led by 5AM Ventures and Foresite Capital with participation from Corner Ventures. Padhi wouldn’t lay out how much of a runway the $135 million affords but expects it would allow the company to complete the ongoing phase 1 trial and a future phase 2 acute pain study while planning for the chronic pain study. He also said Latigo was planning to initiate a clinical trial for the second molecule in the “near future.”
같은 날 Biospace의 기사는 좀더 약물의 디자인과 관련한 차별점에 방점을 찍고 기사를 송고했습니다. 독성 완화를 위해 peripheral nervous system에만 전달을 하고 Central Nervous System (CNS)에는 약물이 들어가지 않도록 디자인하고 테스트를 했다는 것을 강조하고 있습니다. 뿐만 아니라 Latigo 연구팀이 Amgen에서 오랜기간 개발에 참여한 NaV1.7과의 병용이 중요하다는 얘기도 Yale University의 Waxman 박사의 입을 빌려 어필하고 있습니다.
Latigo Launches into Non-Opioid Pain Medicine Space with $135 Million Series A – Biospace 2/14/2024
Founded by Westlake Village BioPartners in 2020, Latigo is developing non-opioid-based therapeutics against genetically validated targets for pain—and with the opioid epidemic continuing to ravage the U.S., new options are urgently needed. In an interview with BioSpace, Sean Harper, founding managing director at Westlake, noted a dearth of available programs in which to invest.
“When we began to get into this focus of a pain company, we found that . . . it [was] really hard to find any programs that you could license in,” he said. Additionally, he said it was difficult to find people with expertise in the space, “because there’s so little investment and innovation going on in biopharma or academia in pain. It’s kind of shocking.”
Fortunately for Westlake, Amgen—where Harper was previously head of R&D—had recently made the strategic decision to get out of the neuroscience space, which included pain, Harper said. “I knew the pain research unit . . . had these fabulous drug hunters with deep expertise in pain, and when they were laid off, we swooped in and we hired them and assembled this team.”
‘Best-in-Class’ Potential
Like Vertex’s VX-548, Latigo’s lead program, LTG-001, is a selective NaV1.8 inhibitor. “There definitely is room for multiple agents targeting NaV1.8,” said Stephen Waxman, a professor of neurology at Yale School of Medicine who previously consulted for Latigo. “At a minimum, there are going to be small nuances of difference.”
While hitting the primary endpoint of significant reduction of pain intensity from 0 to 48 hours in two Phase III trials, VX-548 missed a key secondary endpoint—superiority to Vicodin—and analysts also questioned the drug’s performance in another secondary endpoint: median time to pain relief. VX-548’s time-to-onset had a “more rapid onset to meaningful pain relief” than placebo, Vertex reported, with median time to pain relief being two hours in patients following abdominoplasty (tummy tuck) and four hours in bunionectomy patients versus eight hours for the placebo group.
In its press release, Latigo stated that LTG-001, currently in Phase I trials, has the potential for rapid onset. Harper said VX-548’s time-to-onset “may not be entirely a characteristic of the target. . . . It may be partially due to the particular characteristics of the compound, and we hope that the particular characteristics of our compound could result in faster time to onset.”
Desmond Padhi, interim CEO of Latigo, said the team believes LTG-001 has the potential to be best-in-class. “We’re very focused on making sure that we get optimal biodistribution of our compounds to the tissue of interest . . . the peripheral nervous system where the target is expressed, and minimizing exposure in tissues where the target is not expressed,” the central nervous system (CNS).
But Waxman noted that the jury is still out on the benefits of specifically targeting the peripheral nervous system. He pointed to research suggesting that another sodium channel, NaV1.7, must be blocked within the spinal cord in order to get adequate pain relief. This question has not been raised regarding NaV1.8, he said, but “whether peripheral sequestration of the NaV1.8 blocker is an advantage or not, I think, is still up for grabs.”
Alongside Latigo and Vertex in the pain space, Orion Pharmaceuticals is developing also developing a NaV1.8 inhibitor, and Virpax Pharmaceuticals is looking at other targets.
Harper said he has been interested in the pain space for a long time but has been met a “huge amount of resistance” with people noting the very genericized market. “Of course, that’s what happens when there’s been no innovation for 30 years. Everything’s off-patent.”
“I believe that . . . you have to be a little bit of a contrarian, and kudos to Vertex for having the first real breakthrough here to get it into proof of concept in humans,” he said. “It’s huge for patients.”
같은 날에 나온 Biopharmadive의 기사는 Vertex 약물이 통증 완화에 몇시간이 걸린다는 약점을 파고드는 디자인이라는 것에 방점을 찍고 있습니다. 하지만 Vertex도 Best-in-class 약물 개발이 진행 중이라는 점을 잊지 않고 있습니다.
Hunting a non-opioid painkiller, a biotech reveals plans to chase Vertex – Biopharmadive 2/14/2024
Vertex’s results provided clinical validation of a hypothesis that was already well supported by genetic data. They also set a high bar for success. And with a drug ready for regulatory review, Vertex has established a sizable head start on any competition.
Even so, Latigo claims there’s room to improve on its larger rival’s treatment.
“When a new category opens up like this, somebody makes a first-in-class molecule. Occasionally, that’s the best-in-class, but most of the time something else comes along that is more refined,” said Harper. “So we’re really focused on the differentiation that we can bring to the table.”
Latigo’s lead drug, dubbed LTG-001, is currently in a Phase 1 study. The company is preparing to advance it into a mid-stage trial in acute pain, such as after bunion or stomach surgeries. Testing in chronic pain is also part of the plan, said Desmond Padhi, interim CEO and an operating partner at Westlake.
According to Padhi, preclinical data has shown LTG-001 to be highly selective for NaV1.8, avoiding penetration into the brain and the side effects that would go with it. He also noted early safety data suggesting Latigo could explore a wide range of doses.
Selectivity was also prioritized by Vertex’s chemists, who crafted a molecule that’s at least 30,000 times more selective for NaV1.8 than eight other sodium channels. The Massachusetts company is working on successor molecules, too.
Harper describes such selectivity for NaV1.8 as “table stakes” for companies who, like Latigo, hope to follow Vertex. Still, he noted questions that remain unanswered, such as whether the several hours it took for Vertex’s compound to provide relief in testing reflect the Nav1.8 approach or the drug.
“We think it’s possible that could be a molecule attribute,” said Harper. “We think it’s possible that you could get a faster onset with a different molecule, along with perhaps more efficacy by being able to push dose safely in the chronic setting.”
Latigo, which currently has 26 employees, has to prove that in testing. But investor interest is high, said board chair and Neuron23 CEO Nancy Stagliano, giving Latigo options for further fundraising.
“I do think the potential here is to raise capital via a number of vehicles,” said Stagliano. “It’s now going to be a question of what the company views as the best situation.”
Endpoints News에서는 좀 색다른 것을 적고 있는데, Sean Harper가 Drug Pricing에 대해 경험한 일을 나누고 있습니다. 과거 Migraine 약물인 Avvig을 개발할 때 경험을 토대로 만성 통증 완화 치료는 Opioid로 거의 불가능하기 때문에 이러한 Unmet Medical Need를 만족하는 Non-Opioid 약물의 가격적 Merit이 있다는 것을 얘기하고 있습니다.
The company uses computer-assisted structural-based drug design to “optimize the selectivity, potency and biodistribution” of its compounds, Padhi said. In Nav1.8, Latigo is making sure its drug gets into the peripheral nervous system while avoiding the central nervous system.
During his days at Amgen, Harper was confronted with the realities of drug pricing, a key factor that Vertex, Latigo and other biotechs will have to contend with as they develop non-opioid pain medicines. He pointed to his experience developing biologics for migraine prophylaxis, describing migraine as a “different kind of pain syndrome.”
“Trying to treat patients with chronic pain with opioids is really virtually impossible, so you end up with a lot of patients who you send home with nothing that works. There’s an enormous opportunity,” Harper said.
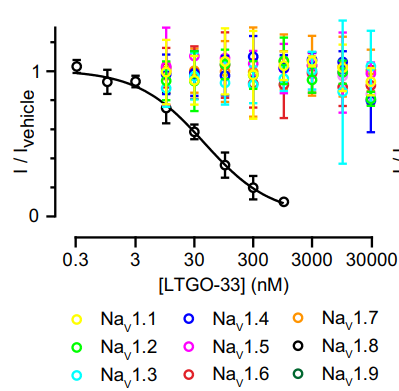
올해초에 Latigo Biotherapeutics의 Bryan Moyer 팀은 LTGO-33이라는 약물에 대한 연구결과를 Molecular Pharmacology에 발표했습니다. LTGO-33의 구조는 아래와 같습니다.

NaV1.1을 비롯한 8개의 타겟에 비해 NaV1.8에 대한 선택성이 600배 이상입니다. LTG-001은 LTGO-33에서 보다 Lead Optimization 된 약물일 것이니까 선택성이나 약효면에서 좋다는 가정을 할 수 있겠습니다. Non-Opioid Pain Medicine 개발에서 과연 ex-Amgen vs Vertex의 경쟁이 어떻게 판결나게 될지 흥미진진합니다.