안녕하세요 보스턴 임박사입니다.
지난 수년간 mRNA Translation을 Small Molecule로 조절하려는 노력이 활발한데 Small molecule의 가장 큰 장점은 먹을 수 있다는 점 (Oral Delivery)이라고 할 수 있습니다.
BIOTECH (71) – Expansion Therapeutics – RIBOTAC Degrader by RNA-Small Molecule Binding
BIOTECH (126) Accent Therapeutics: Small Molecules Targeting RNA-Modifying Protein
그런데 Oligonucleotide 자체가 Oral Delivery가 된다면 어떨까요? mRNA Translational Regulation에 Clinically validated regimen은 Antisense Oligonucleotide 나 siRNA인데 RNA 기반인 siRNA에 비해 DNA gapmer를 보유한 Antisense Oligonucleotide는 Formulation Development에 따라 Oral Delivery가 될 가능성이 여전히 있어 보입니다. 다른 Tide 약물인 Peptide 약물이 최근 GLP-1 치료제 분야에서 경구용으로 성공하고 있는 것을 보면 더욱 이런 가능성을 간과하기 어려운데요.
그래서 Oral Antisense Oligonucleotide에 대한 생각을 좀 해보고자 합니다. 이것에 대해 Novartis 연구원이면서 블로거인 Derek Lowes의 글에서 시작해 볼까 합니다. 2017년에 쓴 글인데요 이렇게 오래 전의 글도 다시 읽어볼 수 있다는 게 역시 블로그의 매력이라 할 수 있습니다.
Mongersen Fails – In The Pipeline by Derek Lowe 10/23/2017
Readers may recall a post here last year about an odd trial of an antisense drug for Crohn’s disease. Celgene had acquired the drug (mongersen, GED-301) from Nogra Pharma of Ireland back in 2014 as a late-stage candidate, and for a while, things looked good. In fact, going back and reading the stories, you’d think that everything was pretty much on track:
Celgene ($CELG) bet big on the little-known Irish biotech Nogra Pharma when it partnered on a mid-stage drug for Crohn’s disease. And today Celgene spelled out the reasons why it gambled $710 million upfront on a Phase II drug, highlighting data that support a clear case that the therapy can help spur clinical remission in a broad group of patients.
An oral antisense agent is a pretty bold move, but then again, a Crohn’s drug of that sort just needs to hit its target in the gut wall, not make it into circulation. And mongersen’s target is Smad7, a key player in the transcriptional signaling machinery for an important inflammation pathway (among other things). This is the sort of target that is very difficult indeed to go after with a small molecule, and that’s when you see the antibodies and oligonucleotide-based modes get tried.
Later results were not as encouraging, though. And the reason I called that trial uninterpretable almost exactly a year ago was that it had no control arm, making it very hard to tell the difference between mongersen’s effects and a placebo. On Friday, Celgene had an announcement on a full placebo-controlled Phase III trial, and guess what? It actually isn’t that different from a placebo. Fancy that. The announcement was that the trial was being discontinued due to futility; an interim analysis showed that nothing was happening.
Unfortunately, that candidate was actually a pretty important part of Celgene’s plans and revenue projections. When the company did their 2014 deal, it raised eyebrows because of the steep upfront price for a relatively unproven therapy from a relatively unknown (and very small) partner, but Celgene was (as they said at the time) into Planning Boldly For the Future, as well as Executing Transformative Deals on Late-Stage Clinical Assets and all that stuff. Unfortunately, the science crept up and sank its teeth into the ankle of this mighty deal, and one would assume that mongersen itself is no more. There’s a lot of finger-pointing about putting that much money into something so thin, but of course if the compound had worked, everyone would be taking visionary dealmaker victory laps. It’s evaluating that “if the compound works” part that is the tricky part, and a tiny company’s oral antisense agent for Crohn’s was always going to be a gamble. You just wonder if it had to be quite as expensive a gamble as it was.
And as always, whenever something like this happens, I will remind people that this is why you run big, well-controlled Phase III trials. Back in Phase II, mongersen looked as if it were going to work (as that quote above illustrates). It doesn’t seem to have any particularly bad safety issues, so under some regulatory proposals, that would have been the time to let suffering Crohn’s patients take it on a risk basis, speed up development, get the regulatory barriers out of the way, all that stuff. But that would have given everyone three years of useless placebo, at a no doubt stiff price. And since more drugs in clinical trials fail than work, I’m still baffled at how giving people a chance to pay for them at that point is supposed to improve health or save anyone money. It certainly wouldn’t have in this case. Celgene stuffed well over $700 million in real money into the shredder on this effort, and a million Crohn’s patients could have joined them.이 블로그 글이 올라온 지 7년여가 지났지만 어찌된 영문인지 Nogra Pharma의 홈페이지에는 여전히 Mongersen의 임상3상이 진행 중인 것으로 나옵니다.
2015년 New England Journal of Medicine에 Mongersen의 임상2상 결과가 나와 있습니다.
Introduction:
The objective was to assess the efficacy and safety of GED-0301, an antisense oligodeoxynucleotide to Smad7, in active Crohn’s disease (CD).
METHODS:
This phase 3, blinded study randomized patients (1:1:1:1) to placebo or 1 of 3 once-daily oral GED-0301 regimens: 160 mg for 12 weeks followed by 40 mg continuously or alternating placebo with 40 or 160 mg every 4 weeks through week 52.
RESULTS:
In all, 701 patients were randomized and received study medication before premature study termination; 78.6% (551/701) completed week 12, and 5.8% (41/701) completed week 52. The primary endpoint, clinical remission achievement (CD Activity Index score <150) at week 12, was attained in 22.8% of patients on GED-0301 vs 25% on placebo (P = 0.6210). At study termination, proportions of patients achieving clinical remission at week 52 were similar among individual GED-0301 groups and placebo. More placebo vs GED-0301 patients achieved endoscopic response (>50% decrease from baseline Simple Score for CD) at week 12 (18.1% vs 10.1%). Additional endoscopic endpoints were similar between groups at weeks 12 and 52. More placebo vs GED-0301 patients had clinical response (≥100-point decrease in the CD Activity Index score) at week 12 (44.4% vs 33.3%); at week 52, clinical response rates were similar. Adverse events were predominantly gastrointestinal and related to active CD, consistent with lack of clinical and endoscopic response to treatment. Two deaths occurred (GED-0301 total group) due to small intestinal obstruction and pneumonia; neither was suspected by the investigator to be treatment-related.
DISCUSSION:
GED-0301 did not demonstrate efficacy vs placebo in active CD.
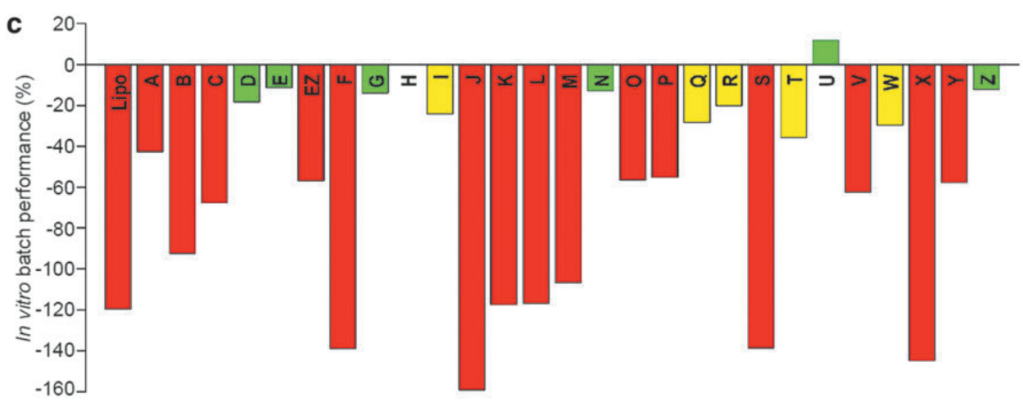
Batch 별로 SMAD7 in vitro test를 했을 때 약효가 크게 차이가 나는 것을 관찰했습니다. Green (best performance batches), Yellow (decreased performance batches), Red (poor performance batches)의 세가지 군으로 나눠보았을 때, Red의 것이 크게 낮은 것을 알 수 있습니다.
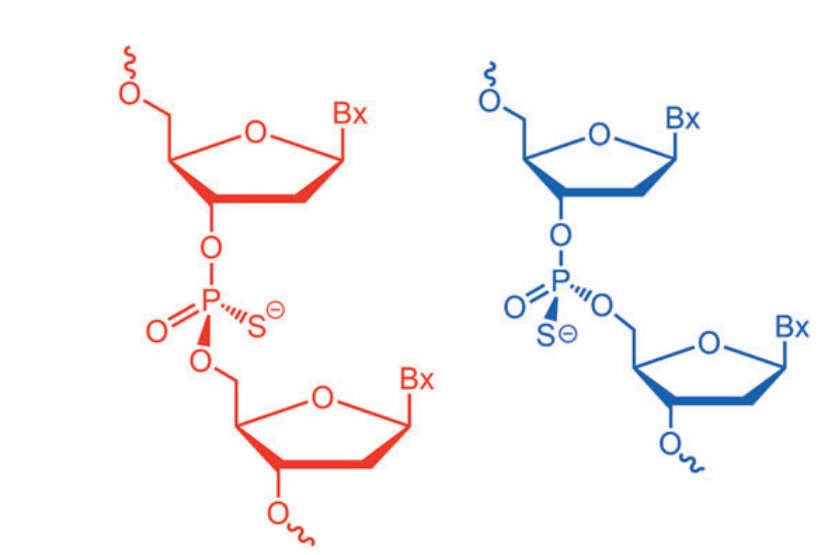
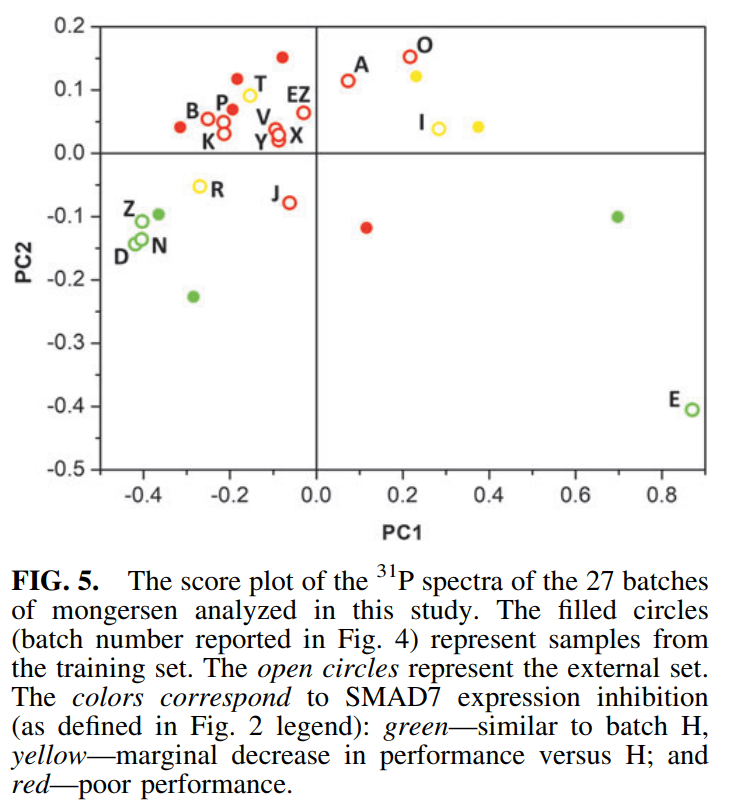
그리고 그 이유로 13P NMR을 측정해 본 결과 Batch 별로 Thiophosphates의 Chirality가 다르게 분포한 것을 발견한 것입니다. Green (best performance batches), Yellow (decreased performance batches), Red (poor performance batches)의 세가지 군으로 나눠보더라도 위치가 크게 차이가 나는 것을 알 수 있습니다. 따라서 Nogra Pharma의 결론은 Chirality of thiophosphates가 Critical Quality Attributes (CQAs)라는 것입니다. 흥미로운 관찰입니다.
Mongersen은 21-nt Oligonucleotide인데 (3′-5′)d(P-thio)(G-T-m5C-G-C-C-C-C-T-T-C-T-C-C-C-m5C-G-C-A-G-C)로서 세번째 C와 17번째 C는 5-meC이고 모든 Linker는 Thiophosphate Bonds로 되어 있어서 실제로 N20 개의 Stereoisomers가 가능합니다.
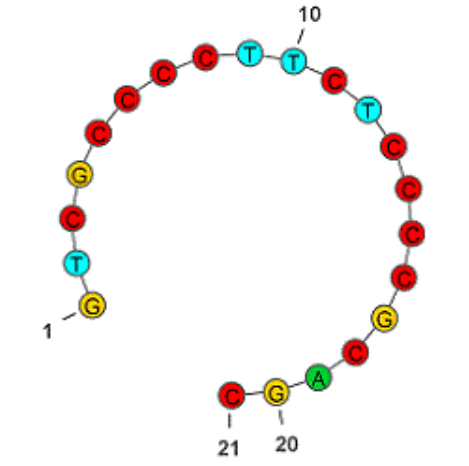
이 결과에 대해 2023년 Pharmaceutics에서 이탈리아 과학자들은 Monsergen의 임상2상과 임상 3상 데이타가 큰 차이가 난 이유는 Thiophosphates Stereochemistry에 대한 Batch-by-batch variability 때문이라고 하는 논조를 냈습니다. 이 약물이 개발될 당시에는 Thiophosphate bond의 Chirality를 Control하는 Synthetic methods가 없었는데요 하지만 이제는 다양한 방법들이 존재합니다.
예를 들면 Wave Life Sciences가 바로 Thiophosphate Stereocontrol을 Platform 기술로 하는 회사입니다.
BIOTECH (8) – Wave Life Sciences의 RNA Editing 신약 가능성에 GSK가 투자하다.
Scripps Institute의 Phil Baran Group도 BMS와 함께 Phosphate chirality control 방법을 개발한 바가 있습니다.
Nucleoside (5) – Phil Baran의 Stereochemical Synthesis of Nucleoside Triphosphates
이외에도 Antisense Oligonucleotide 기술이 지금은 훨씬 진화해서 아마 새로운 디자인을 해야할 것으로 보이지만 Oral Antisense Oligonucleotide Drugs에 대한 가능성은 아직 개발 가능성이 남아있다고 볼 수 있을 것 같습니다. Mongersen은 아마 결국 다시 사용될 수는 없을 것 같습니다. 이 결과를 인정한다고 하더라도 어떤 Stereocontrol을 해야할지에 대한 연구도 필요하고 또한 Stereocontrolled Antisense Oligonucleotide 생산은 훨씬 비싸서 투자자를 만나기도 아마 어려울 것 같습니다. 그래도 한가지 알게 된 사실은 임상2상에서 성공하고 임상3상에서 실패한 경우 그냥 지나칠 것이 아니라 이와 같이 원인 규명이 반드시 필요하다는 것입니다. 기술진보는 계속되어 언젠가 Oral Antisense Oligonucleotides의 시대가 오기를 기대합니다.
