안녕하세요 보스턴 임박사입니다.
Covalent DNA-Encoded Library는
Totus Medicines is making a play for the PI3Kα inhibitor market. Exiting stealth with a $40 million series A round, the Massachusetts-based biotech plans to advance a molecule designed to improve on existing PI3Kα inhibitors such as Novartis’ Piqray.
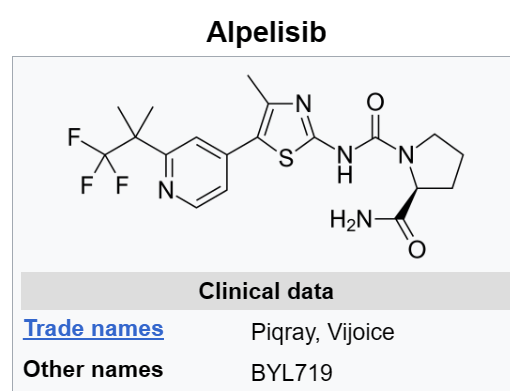
Piqray (Alpelisib)
PI3K, in both its alpha and delta forms, is a long-standing target of interest to oncology drug developers. With studies implicating overactivity of the kinase in multiple cancer types, researchers have delivered a series of approvals ranging from Gilead Sciences’ Zydelig to TG Therapeutics’ Ukoniq. Many of the drugs hit the delta form of kinase, but Piqray is specific to PI3Kα.
Totus references the competition in the statement to disclose its $40 million series A round, arguing that there is an opportunity for its asset because, after spending “decades and billions of dollars,” the pharma industry has only achieved “moderate success in <10% of patients with PI3Kα-mutant cancers.”
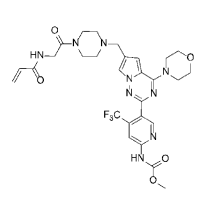
Armed with cash from a syndicate led by DCVC Bio and Northpond Ventures, Totus wants to improve on the performance of existing PI3Kα inhibitors. Totus’ belief that it can do so is underpinned by preclinical studies in which its lead therapy, TOS-358, showed efficacy in PI3Kα-mutant tumor types that are largely unaffected by existing molecules.
Phosphoinositide 3-kinase alpha (PI3Kα) is the most frequently mutated oncogene in cancer, inferring a critical role for this protein in neoplasia. The molecular biology of PI3Kα reveals it to be a protein that integrates a large and diverse set of cellular signals. In the development of small molecule inhibitors of this target protein it has been demonstrated that deep but also durable inhibition is critical to potent anti-cancer activity, an area traditionally challenging for reversible competitive and allosteric inhibitors. TOS-358 was developed to inhibit covalently both wild-type and mutant PI3Kα with observed IC50 s of 2.2 nM for WT PI3Kα and 4.1 nM for mutant PI3Ka (H1047R). TOS-358 is highly selective in a kinome-wide screen and selective for PI3Kα over other isoforms. We confirmed covalent binding to PI3Kα by NanoBRET in washout experiments, where it was observed that TOS-358 maintained 90% binding at 100 nM 6 hours after washout; in contrast, binding of Alpelisib at 100 nM was completely lost over the same time frame. Irreversible binding was further established using TR-FRET assay in which Kinact/KI was found to be 5.6 × 107 M−1s−1. Importantly, we also noted that TOS-358 produced sustained inhibition of phosphorylated AKT(S473) to 48 hours while allosteric inhibitors lose >60% inhibition. TOS-358 mediated cell growth inhibition has been evaluated in a panel of 120 cell lines and compared with Alpelisib in the same panel. This analysis revealed approximately 50% more cell lines to be responsive to TOS-358 compared with Alpelisib. There was no strong association of response and PI3Kα mutation status, and in fact for both TOS-358 and Alpelisib there was a higher frequency of WT PI3Kα cell lines responding to either treatment. TOS-358 activity has been tested in multiple different cell-derived and patient-derived xenograft cancer models and was found to produce reproducible and substantial tumor growth inhibition. Indeed, in several PDX models, TOS-358 induced tumor regressions while the clinical stage molecules Alpelisib and Inavolisib were unable to generate similar tumor regressions despite pharmacokinetic exposures comparable to, or in excess of, TOS-358. Finally, TOS-358 generated little or no glucose impact in mice and dogs at exposures that produced superior efficacy in these cancer models. Taken together, our in vitro and in vivo data reveal TOS-358 to be a potent, selective covalent inhibitor of PI3Kα with superior anticancer activity to comparator molecules.
According to Totus, TOS-358 induces tumor regressions in models of breast, colon, lung and stomach cancers, while other PI3Kα inhibitors only have “mild effects.” Totus will start showing whether the effects of TOS-358 translate into humans when it takes the candidate into the clinic in the second half of next year.
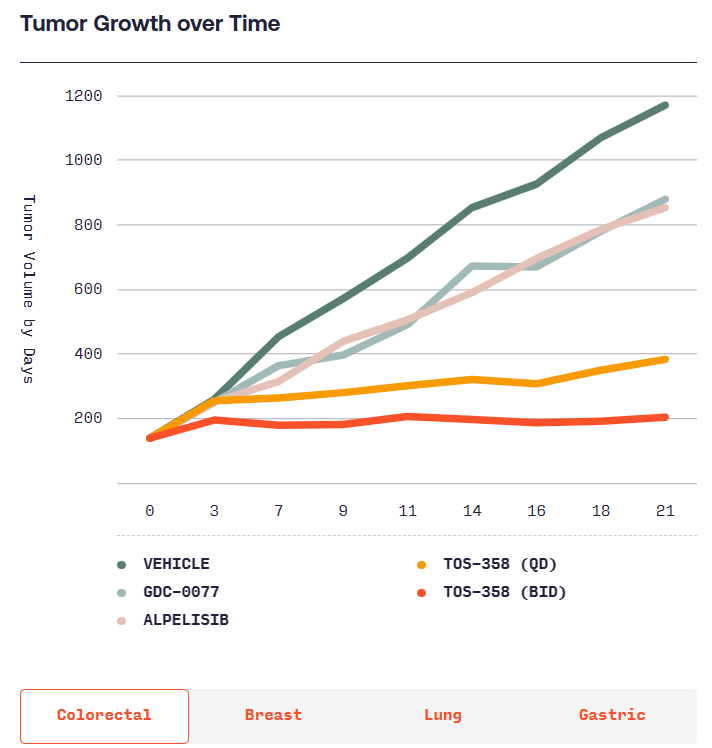
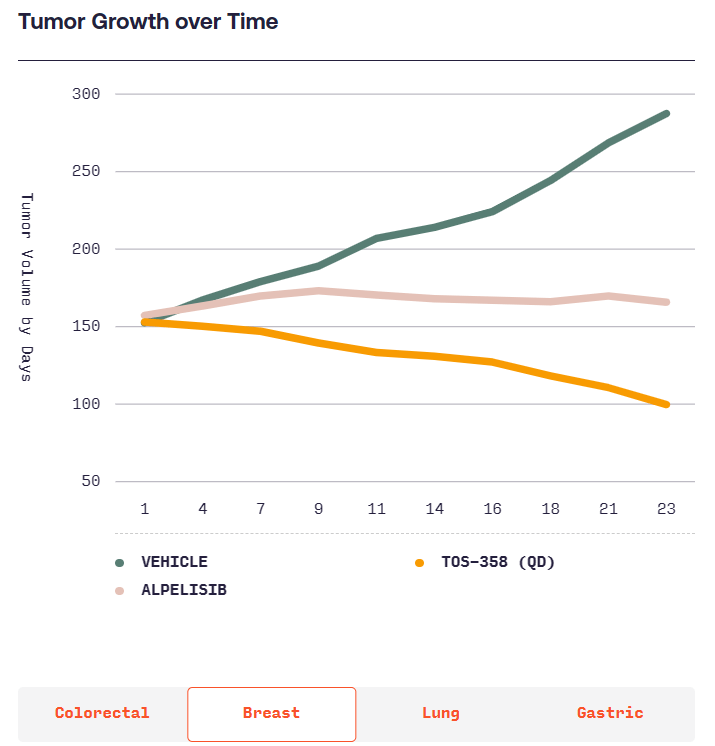
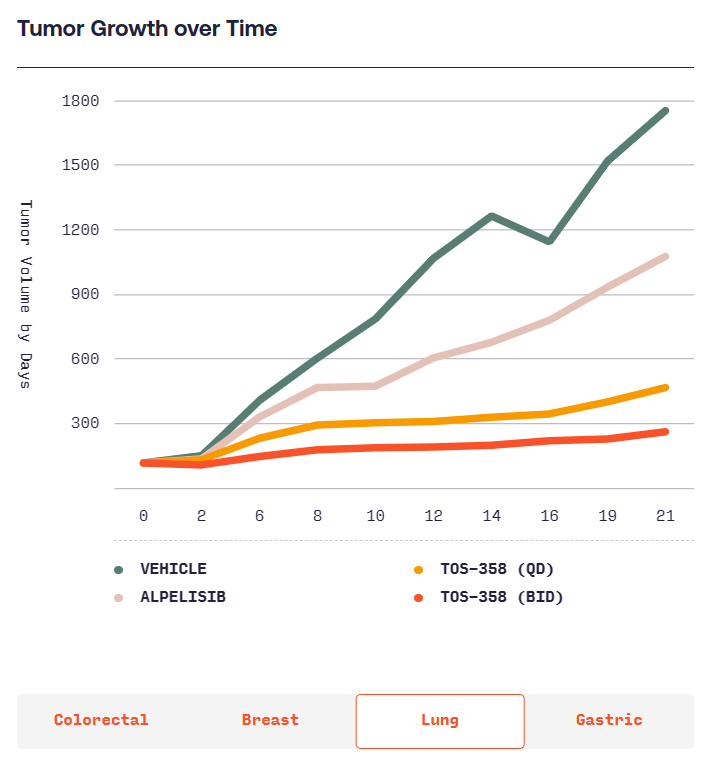
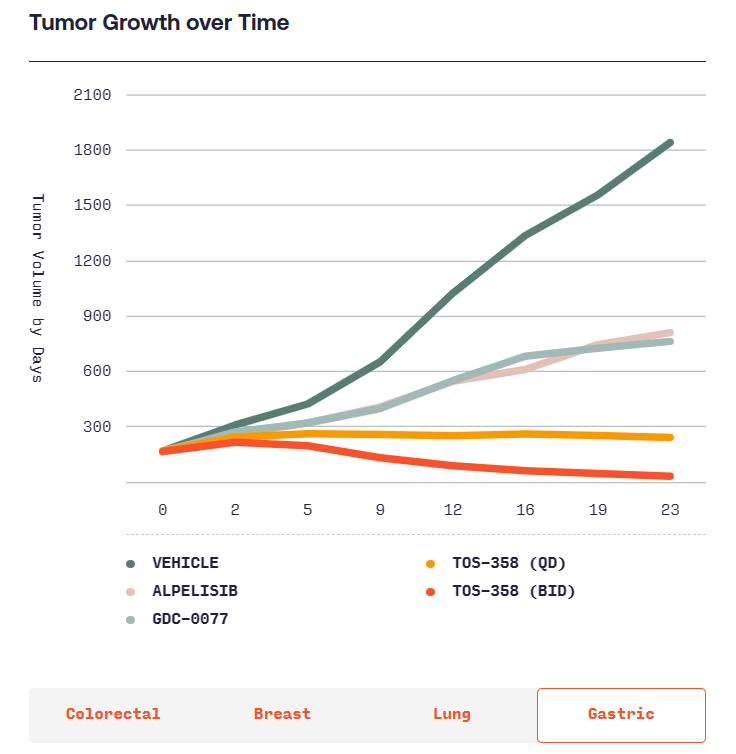
(Source: https://www.totusmedicines.com/pipeline#colorectal)
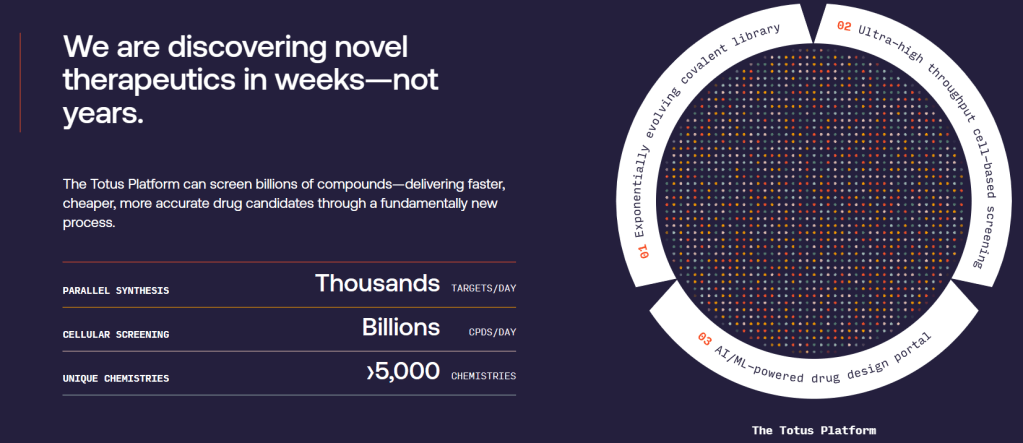
The candidate is the product of a platform that uses molecular tags that track drug binding in individual cells. Using the platform, Totus claims it can screen billions of drug molecules across thousands of genes in parallel, enabling it to find hits against high-value targets.
“Our drug discovery platform is capable of creating treatments for previously untreatable diseases. We are drugging undruggable targets at scale, moving us closer to a world where every physician and every patient can look forward to effective treatments for the most devastating illnesses,” Totus CEO and co-founder Neil Dhawan, Ph.D., said in a statement.
Earlier in his career, Dhawan co-founded Dual Therapeutics, a startup that set out to simultaneously block two important and common cancer signaling pathways using a small molecule. The biotech landed a deal with Bristol Myers Squibb but then disappeared from view.
Phase 1 Trial of TOS-358 for PI3Kα-Mutated Tumors Begins Dosing – Targeted Oncology 4/18/2023
The first patient in a trial of TOS-358, a first-in-class covalent PI3Kα inhibitor, in patients with various solid tumors with known PI3Kα mutations, began treatment according to a press release from Totus Medicines.1
The multicenter phase 1 TOS-358-001 trial (NCT05683418) evaluating the safety and efficacy of TOS-385 includes 2 parts: a dose escalation portion and a dose expansion portion using the recommended phase 2 dose. Investigators will enroll an estimated 241 patients with PI3Kα-mutated tumors including colorectal cancer, gastric cancer, HER2-negative breast cancer, lung cancer, and endometrial cancer.
PI3Kα, which is mutated in approximately 15% of all cancers, can be targeted with PI3K signaling pathway inhibitors, but current pathway inhibitors lack specificity, leading to toxicity, and cannot achieve continuous greater than 95% inhibition needed for best antitumor efficacy.2 TOS-358 is the first highly specific covalent inhibitor of PI3Kα, which demonstrated durable near 100% inhibition of PI3Kα activity in preclinical studies.
The phase 1 portion of the study uses a 3+3 dose escalation design to determine the minimum effective dose and maximum tolerated dose.1 TOS-358 will be investigated orally as once daily or twice daily doses. The phase 1b portion will enroll patients with PIK3Cα mutations or amplifications in separate cohorts based on tumor type including colorectal, gastric, non–small cell lung, breast, squamous cell carcinoma of the head and neck, urothelial and selected gynecological cancers (ovarian cancer, cervical cancer, or endometrial cancer).
Eligible patients must have a PIK3Cα mutation or amplification and have received no prior treatment with PI3K, AKT or mTOR inhibitors, except for patients with breast cancer. They could not have recent systemic anticancer treatment prior to the start of treatment. Additionally, patients with central nervous system metastases were excluded.
The primary end points are dose-limiting toxicities within the first 21 days of treatment and the incidence and severity of adverse events from the start of treatment until 30 days after patients receive the last dose.
Using cellular analysis based on proprietary molecular tags that track drug binding in individual cells, Totus’s Accel™ Platform led to the rapid drug development of TOS-358. This enabled the company to file an investigational new drug application 18 months after the drug’s discovery.
In preclinical animal model studies, TOS-358 was shown to potently and specifically lead to deep and durable PI3Kα inhibition.2 It did not lead to significant hyperglycemia in mice, rats, and dogs at efficacious dose levels, unlike reversible PI3Kα inhibitors. When compared with previous ATP-competitive and allosteric reversible PI3Kα inhibitors across over 30 different PDX and CDX mutant PI3Kα dependent cancer models, TOS-358 demonstrated superior efficacy.
“TOS-358 represents a promising new approach to the treatment of the root cause of nearly 15% of all cancers, and we are excited to be able to advance it into clinical development at such an accelerated rate,” said Neil Dhawan, PhD, chief executive officer and co-founder of Totus Medicine, in a press release.1
REFERENCES
1. Totus Medicines announces first patient dosed in phase 1 trial of TOS-358 for the treatment of select solid tumors. News release. Totus Medicines. April 13, 2023. Accessed April 17, 2023. https://prn.to/3KLRwZX
2. MacDougall JR, Bradley J, Mak R, Dhawan N, Chen W. TOS-358, a first-in-class covalent PI3Kα inhibitor, demonstrates superior efficacy and does not induce significant hyperglycemia at efficacious doses in multiple animal models. Cancer Res. 2023;83(7_suppl):4946. doi:10.1158/1538-7445.AM2023-4946
Totus Medicines, a company revolutionizing small molecule drug discovery and development using covalent libraries and AI tools, announced today that Nassim Usman, Ph.D., has been named President & Chief Executive Officer and closed a $66M Series B financing led by DCVC Bio. Neil Dhawan, Ph.D, Totus co-founder, founding CEO, and CSO, will transition into a new role as CSO and Head of R&D, where he will continue to oversee the company’s platform, programs, and data.
“We are thrilled by Nassim’s arrival,” said DCVC Bio Managing Partner John Hamer. “He brings exceptional experience and acumen to a company poised to bring much-needed therapies to market. I’m equally excited by the fact that Neil – whose vision and determination have brought Totus to where it is – will continue to contribute at a senior level to the company’s rapid growth.”
“Nassim is exactly the right leader for Totus,” said Dhawan. “His extensive drug development background and vision for the Totus platform will help lead us through the next phase of growth for the company. His strong management experience will help shape Totus as we continue to advance breakthrough therapeutics, and just as importantly, Nassim embodies our culture and values.”
Before joining Totus Medicines, Dr. Usman served as President, CEO and Board member at Catalyst Biosciences (NASDAQ:CBIO, now Gyre Therapeutics, NASDAQ:GYRE). Dr. Usman has an extensive background in C-Suite management (CSO, COO, CEO and Principal Financial Officer), Board membership on and venture capital investing (Morgenthaler Ventures) in several private and public companies including Sirna Therapeutics (acquired by Merck) and Principia Biopharma (acquired by Sanofi). Dr. Usman currently serves on the Board of GYRE and the advisory boards of two private biotechnology companies. During his career, he has advanced several drugs into clinical development, executed multiple licensing deals, and raised capital through private and public financings. Dr. Usman received his B.Sc. and Ph.D. in Organic Chemistry from McGill University and completed a post-doctoral fellowship at MIT.
“Neil and the entire team at Totus have built an extraordinary a platform that has the potential to transform small molecule discovery and have built a pipeline of clinical candidates led by TOS-358, a covalent inhibitor of PI3Ka in the clinic,” said Dr. Usman. “I am delighted to join Neil, our investors and the team as we build out the platform and advance our clinical development pipeline.”
The company closed the final tranche of a Series B financing totaling $66 million, led by DCVC Bio and including the participation of North Pond Ventures, Camford Capital, and the Regents of the University of Minnesota. Proceeds from the Series B financing will be used to advance Totus’ clinical program, expand the pipeline, and evolve the platform.
About Totus Medicines
Totus Medicines is discovering and developing small molecule medicines using a novel DNA-encoded covalent library technology combined with artificial intelligence/machine learning (AI/ML). With the unprecedented ability to screen billions of drug candidates against thousands of targets simultaneously, the company’s novel platform can find drugs that are dramatically superior to molecules discovered through previous technologies, including drug candidates for currently undruggable targets.
Sitting down with… Neil Dhawan – Drug Discovery World 8/10/2023
Reece Armstrong speaks to Neil Dhawan, the Co-founder and CEO of Totus Medicines about the company’s approach to drug discovery using fundamental technologies in chemistry, biology and artificial intelligence (AI).
RA: How is Totus Medicines attempting to target untreatable or undruggable diseases?
ND: Discovering a highly effective drug is like winning the lottery. If you can buy 100,000 times the number of tickets – or screen 100,000 times as many therapeutic molecules – you’re going to win a lot more and a lot faster. Totus has enabled cellular search across vast chemical space at massive scale to deliver effective medicines at an unprecedented pace. Totus has been able to identify the first highly-effective covalent molecule against the most mutated oncogene, PI3K-alpha, in four months and moved this molecule into clinical trial in two years.
RA: Could you discuss how Totus uses high-throughput cell-based screening, AI/ML & covalent chemistry to improve its drug discovery processes?
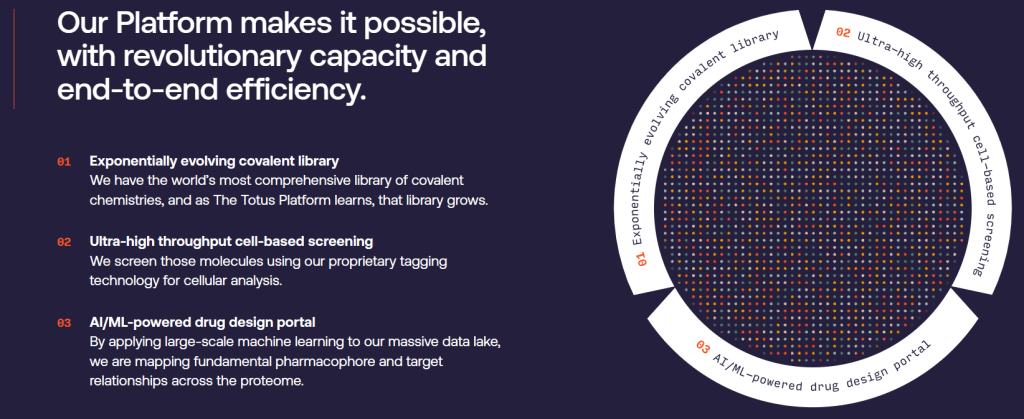
ND: Totus has innovated three interdependent and fundamental technologies in chemistry, biology, and AI to enable screening of billions of candidate molecules against hundreds of targets in parallel. First, we have built a technology to rapidly synthesise billions of highly drug-like compounds. Second, we have created a novel DNA-tracking technology to analyse billions of molecular interaction in a cellular setting. Third, we have created a 3D-AI platform to analyse all of this data and rapidly design highly-effective drug candidates.
RA: How have advancements in technology enabled companies like Totus to begin to target previously untreatable diseases?
ND: For many untreatable diseases like cancer, there are an array of drug targets that we know are driving the disease. However, current technologies have not been able to yield effective molecules to impact these drug targets. Effectively drugging all of these undrugged targets would lead to enormous patient benefit and potentially produce curative options. By enabling a million-fold improvement in drug screening, Totus is rapidly unearthing effective candidate molecules in months against targets that have plagued current technology for decades.
RA: You’ve recently dosed the first patient in a clinical trial of TOS-358, your first-in-class covalent PI3Kα inhibitor for the treatment of numerous cancers. Could you tell us about the trial and TOS-358?
ND: TOS-358 is an orally available, highly selective covalent inhibitor of PI3Kα designed to achieve deep and durable inhibition of PI3K-AKT signalling with no off-target effects. TOS-358 consistently demonstrated efficacy superior to non-covalent PI3Kα inhibitors (ATP competitive and allosteric) in patient-derived and cell line-derived xenograft models with no significant toxicities. The TOS-358-001 study is a multicentre dose escalation Phase I), dose expansion (Phase Ib) clinical study evaluating the safety, tolerability, and preliminary efficacy of TOS-358. Eligible subjects are adults with PI3Kα-mutant tumours. The study is currently enrolling in the US with European sites planned to open later in 2023.
RA: How impactful could TOS-358 be across a range of cancers?
ND: Based on our preclinical studies, TOS-358 is highly effective across PI3Kα-mutant tumours, including breast, colorectal, lung, gastric, head and neck, and ovarian tumours. Our Phase I study (NCT05683418) tests TOS-358 as a monotherapy in cohorts of patients with a range of tumour types.
RA: What have been some of the long-term challenges in targeting harder to treat diseases?
ND: Many hard-to-treat diseases are driven by hard-to-drug targets. While reliable targets may be unclear in certain disease, the genetics revolution has unearthed central and critical targets in a variety of diseases. However, even when we know the important drug targets, finding effective molecules to intervene with that drug target has proved challenging. Totus’ platform is seeking to solve this critical challenge by enabling cellular screening on a million-fold improve scale versus current technologies.
RA: Could you talk more about Totus Medicines’ goal of identifying molecules for every gene in the human genome? How can this impact drug discovery?
ND: It’s impossible to overstate the potential of Totus’ work. If you can screen the whole human genome, you can attempt to drug any relevant target with highly effective and specific drugs. By understanding how your drug is interacting with the full array of human proteins at the same time, we can rapidly deduce safe, effective drugs for almost any target.
RA: With such large volumes of data generated through screening approaches, is data analysis a challenge for Totus?
ND: The Totus platform innovation is especially timely with the emergence of ML, which are very data hungry. At Totus we say, “The more data, the better!”
RA: What’s the future outlook for Totus Medicines?
ND: In 2023 at Totus, we have the opportunity to prove the power of our platform with the most important form of data – clinical data. Through the Totus platform, we have discovered and advanced TOS-358 into the clinic to achieve efficacy for patients with PI3Kα-mutant tumours. 15% of all cancer patients have PI3Kα- mutant tumours, representing one of the most significant unmet needs in all of oncology. Importantly, TOS-358 is just the beginning for Totus. We are building an array of drug programmes targeting other incredibly important targets in oncology.
DDW Volume 24 – Issue 3, Summer 2023
Biography:
Neil Dhawan is the Co-founder and CEO of Totus Medicines, where he focuses on combining small molecule design, structural biology, genetics, biochemistry, and cell biology to design new classes of drugs to target untreatable diseases.
Totus also added industry go-to John Maraganore, the former founding CEO of Alnylam, to its scientific advisory board this fall..
Totus’ lead drug, TOS-358, is a covalent inhibitor of PI3Ka and is in Phase Ia in multiple types of solid tumors. The company is testing once- and twice-daily dosing regimens for the oral candidate.
Once the company collects Phase Ia data sometime next year, it will likely evaluate its next financing route, Usman said. A crossover round is “most likely,” he said, noting Totus’ next key executive hire will be a chief financial officer “as we approach the inflection point where you need one for going public.”
The two dozen-employee biotech could also benefit from R&D collaborations, Usman said. Totus is exploring ways to partner up its machine learning platform to expand into CNS and cardiometabolic diseases. Those two therapeutic areas have seen increasing investor and pharma interest on the backs of neuroscience drug advancements and the explosion of obesity and diabetes treatments.
Elsewhere in oncology, Totus is nearing the development candidate stage for an AKT program and its pipeline also includes work on beta-catenin, which was largely thought to be undruggable but has recently seen a clinical race among biotechs.