(Picture: Maria Grazia Roncarolo, M.D.)
안녕하세요 보스턴 임박사입니다.
Regulatory T cell에 대한 임상연구는 오랜 기간 이루어진 상태인데 Stanford Medicine Prof. Maria Grazia Roncarolo, M.D. 박사팀은 2009년 T reg type 1 cell을 처음으로 발견하였습니다.
2012년 Roncarolo 교수팀은 IL10 expression을 통해서 인간의 CD4+ T cells에서 type 1 regulatory T cell을 발현할 수 있다는 것을 증명하여 Molecular Therapy에 보고하였습니다.
그리고 2014년에 exploratory clinical trials를 통해서 IL-10 allogeneic T cells로 haploididentical- HSC transplanted 환자의 면역적 반응에 대한 임상결과를Frontiers in Immunology에 발표했습니다. 12명의 혈액암 환자에 대해 임상시험을 했는데 T-cell depleted haploidentical hematopoietic stem cell transplantation을 한 다음에 IL10으로 처리한 donor T cell을 넣어주었을 때 5명이 Immune reconstitution이 일어났고 이 중 4명의 환자가 Complete remission이고 면역억제제를 사용하지 않았는데도 7년 이상 생존했다는 결과입니다. 초기에는 graft-versus-host disease (GvHD)가 나타났지만 환자들에게는 큰 영향이 적었다는 것이고 이를 통해서 Tr1 cell therapy의 가능성을 확인할 수 있었습니다.
이러한 연구결과 및 초기 임상결과를 바탕으로 Tr1X를 설립하고 $75 Million Series A를 받았고 올해 TRX 103이라고 명명한 Tr1 cell therapy 약물을 골수이식 후 GvHD 환자에게 투입하는 임상시험을 올해 중으로 시작한다는 계획입니다. 전임상 연구결과는 Molecular Therapy 2017년에 발표를 했습니다.
Tr1X Secures $75M Series A – San Diego Business Journal 1/29/2024
Local biotech firm Tr1X, Inc. secured a $75 million Series A financing to develop therapies to treat and possibly cure autoimmune and inflammatory diseases using specialized types of T-cells – the white blood cells that fight infections. Financing was led by The Column Group, with participation from NEVA SGR and Alexandria Ventures.

Co-Founder & COO
Tr1X Bio
“Our VC investors are confident in our world-class team’s ability to revolutionize the field through our innovative, breakthrough science,” David de Vries, MPhil, Tr1X Co-Founder and COO told the Business Journal. “In just 18 months, the Tr1X team has already demonstrated a solid track record of execution.” He added that the new financing round will help fund the company through most of 2025.
Tr1X is part of a burgeoning clan of startups focusing on the use of regulatory T cells to target various autoimmune diseases. Other notable players include Northern California’s Sonoma Biotherapeutics and Quell Therapeutics, based in London.
With its headquarters in Torrey Pines, Tr1X’s tech is based on Scientific Founder, President & Head of R&D Maria Grazia Roncarolo’s discovery of Type 1 regulatory T (Tr1) cells, which have features that can benefit patients with autoimmune and other inflammatory diseases. The company has developed a method of engineering donated CD4-positive T cells (cells that play a critical role in the adaptive immune system) into ones that mimic naturally occurring Tr1 cells, including protein expression and function.

Scientific Founder, President & Head of R&D
Tr1X Bio
“The ability to develop a pipeline of medicines based on our work on regulatory T cells represents the culmination of decades of discovery and research into the underpinnings of immunological tolerance and autoimmunity,” she shared. “Tr1 cells have unique properties and represent the ideal therapeutic platform to develop ‘immune reset’ therapies.”
Trial Coming Soon
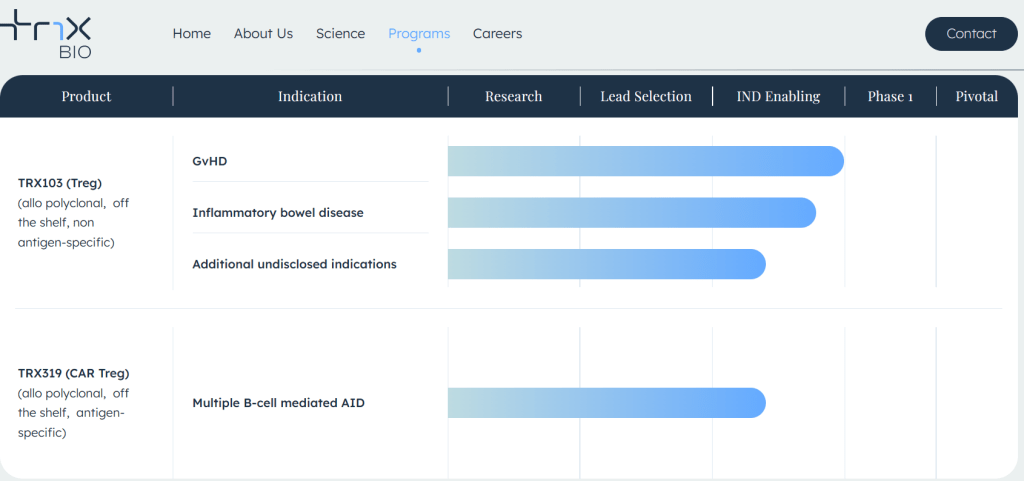
Tr1X is working on its first investigational Tr1 cell therapy — TRX103 — which could treat what’s known as Graft versus Host Disease (GvHD) in patients undergoing mismatched bone marrow transplants. Additional therapies in its development pipeline could treat inflammatory bowel disease, Type 1 diabetes and multiple so-called B-cell mediated autoimmune diseases.
At the helm of the team is CEO Bill Lis, who comes with more than 25 years of biotech executive leadership experience. Lis most recently served as Chairman and interim CEO of Jasper Therapeutics where he led the company’s Series A and follow-on financings and successful brequilimab Phase 1 study readouts in bone marrow stem cell transplantation. Before that, he served as CEO and a Director of Portola Pharmaceuticals (acquired by Alexion Pharmaceuticals) where he built the company from a private research-stage startup into a multi-billion-dollar public company that launched Andexxa and Bevyxxa.
Tr1X is now planning to initiate clinical trials of its TRX103 therapeutic.
“We are preparing to move our TRX103 program into Phase 1 and look forward to updating you on our progress as the study kicks off later this year,” added de Vries. “We hope to be a leader in the field of cell therapies for autoimmune diseases.”
Tr1X Bio
FOUNDED: 2021
CEO: Bill Lis
HEADQUARTERS: 4242 Campus Point Ct. Suite 500
EMPLOYEES: 30
BUSINESS: biotech
FUNDING: $75 million (Series A)
WEBSITE: tr1x.bio
CONTACT: hello@tr1x.bio
NOTABLE: Tr1X’s world-class team has been involved in the development and launch of more than a dozen approved medicines for diseases including RSV, HPV, Metachromatic leukodystrophy, Severe Combined Immunodeficiency (ADA-SCID), Multiple Sclerosis, Psoriasis, DVT and atopic dermatitis.
현재 회사 홈페이지에 나타난 파이프라인은 다음과 같습니다. TRX193을 GvHD뿐만 아니라 IBD 에도 사용한다는 계획이고 CAR-Treg에 대한 약물인 TRX319도 전임상 중에 있습니다.