
(Picture: Stig K. Hansen, Ph.D., former CEO of Carmot Therapeutics)
Sunesis Licenses FBLD Technology To Carmot – Pharmaceutical Business Review 2/7/2010
Sunesis Pharmaceuticals (Sunesis) has granted Carmot Therapeutics (Carmot) an exclusive license to its proprietary Fragment-Based Lead Discovery (FBLD) technology.
Carmot is expected to use the FBLD technology, called ‘Chemotype Evolution,’ for identifying drug candidates in a broad range of therapeutic areas, including inflammatory, metabolic, and neurodegenerative diseases. Sunesis retains full rights to the technology for use in its future internal discovery efforts. Terms of the agreement were not disclosed.
Eric Bjerkholt, senior vice president of corporate development and finance at Sunesis, said: “This agreement reflects our strategy to leverage the value of our non-core assets while focusing our resources on advancing voreloxin into a pivotal Phase 3 trial in acute myeloid leukemia later this year.
“The FBLD discovery technology has the potential to generate compounds which may not be identified through traditional means of drug discovery. We are pleased that we can capitalise on Carmot’s use of the technology to advance its research activities while at the same time retain full rights to use the platform technology for our own internal research applications.”
Leader Ventures Supports Innovative Drug Discovery Company Carmot – Biospace 12/20/2010
Leader Ventures, an investment firm offering blended debt and equity financing, today announced equipment financing to Carmot Therapeutics Inc., an innovative drug discovery company.
Chemotype Evolution, Carmot’s proprietary drug-discovery platform invented by Carmot co-founders Stig K. Hansen and Daniel A. Erlanson while at Sunesis, is being used to identify promising drug candidates in a broad range of therapeutic areas.
“Leader’s flexibility and understanding of our needs has been a tremendous source of support for Carmot, not only financially,” said Stig K. Hansen, CEO at Carmot Therapeutics Inc. “We’re looking forward to continuing to work with Leader Ventures as we grow and lead innovation in drug discovery.”
“The leadership team is what first attracted us to Carmot,” said Brian Best, managing director at Leader Ventures. “We then learned about the Chemotype Evolution technology platform making rapid identification of compounds for a wide variety of challenging disease targets a reality.”
About Carmot Inc.
Carmot Therapeutics was founded by Drs. Hansen and Erlanson in 2008 with the goal of commercializing and further developing Chemotype Evolution. Carmot has licensed the technology and fragment libraries from Sunesis, and Carmot is in the process of further expanding the capabilities of Chemotype Evolution. Chemotype Evolution is a patent pending technology that was invented by Drs. Stig K. Hansen and Daniel A. Erlanson while they were working at Sunesis Pharmaceuticals.
Tackling challenging targets with Chemotype Evolution – Michael’s Bioinformatics Blog 3/26/2014
Carmot Therapeutics, a small company located in San Francisco’s Mission Bay, has developed a very innovative drug discovery technology, called Chemotype Evolution (CE), that relies on fragment-based discovery but is different from traditional FBDD and HTS approaches in important ways.
The first important innovation is that CE relies on a “bait” molecule as a starting point for screening. The bait can be a known ligand, cofactor, or inhibitor. The bait is then derivatized with a linker moiety that allows it to become chemically bonded with every fragment in a proprietary library. This process generates a screening library that contains thousands of bait-fragment hybrids. These hybrids are then screened against the target for binding using either biophysical or biochemical screening techniques in a high-throughput plate format.
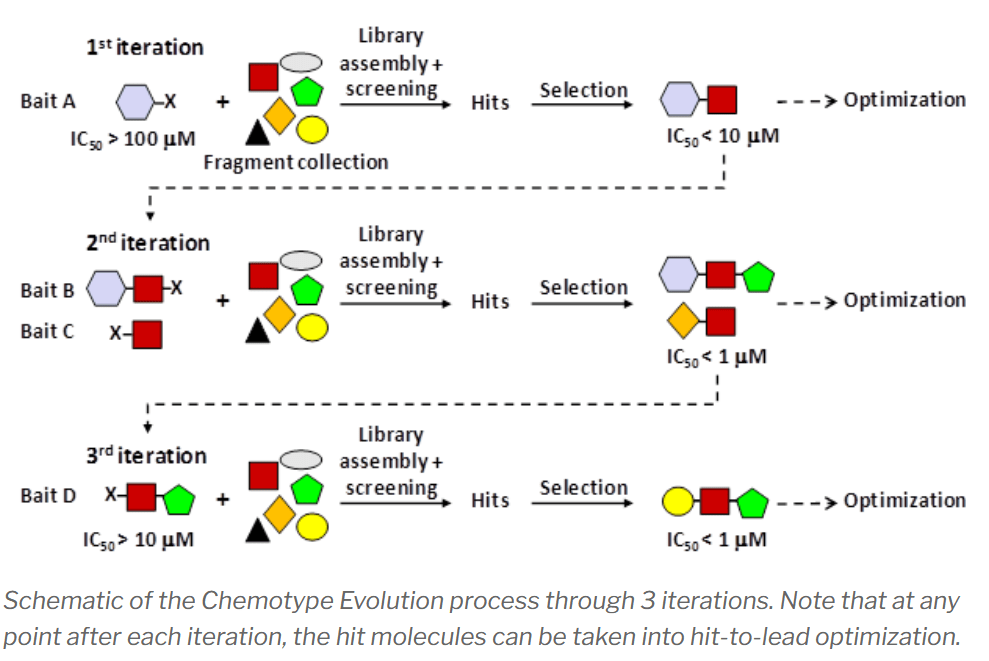
The most powerful aspect of CE is the ability to iterate over chemical space, allowing access to an exponential number of possible fragment-bait hybrids. The method can be iterated with new “baits” derived from the best fragment hits of the previous round. Thus, instead of having 7,000 fragments in your library, after 3 iterations you access 7,000^3 possible combinations (343 billion possible compounds), selecting only the most target-relevant chemotypes at each stage.
The CE approach is similar in concept to the “tethering” approach pioneered at Sunesis, but differs in the fact that no protein engineering of cysteine residues needs to be performed. The bait molecule performs the role of the engineered cys, providing a “handle” that binds to the target and selects for complementary fragment binders.
Carmot Therapeutics just embarked upon their first major industry collaboration with the January 2014 announcement of a partnership with Amgen to use CE technology against two challenging targets. Identifying leads and developing hits will be carried out jointly between the companies, while clinical trials will proceed at Amgen. I think Carmot is definitely a company to watch given its innovative and potentially paradigm-shifting discovery technology and increasing interest from big pharma.
Carmot Therapeutics에 투자한 VC 중 하나인 Axial VC가 Roche와 합병이 발표된 후 블로그를 쓴 것이 있습니다. Chemotype Evolution은 3번만에 1 Billion 이상의 화합물 라이브러리를 만들 수 있는 장점을 가지고 있습니다.
Carmot Therapeutics: Surveying great inventors and businesses- Axial VC Blog 12/26/2023
Carmot Therapeutics is focused on developing novel therapeutics for metabolic diseases like obesity and diabetes. The company’s proprietary drug discovery platform, Chemotype Evolution (CE), allows for the rapid discovery and optimization of small molecule drug candidates.
Obesity and diabetes are two of the most prevalent metabolic diseases globally, affecting over 750M people worldwide. These diseases significantly increase the risk of other severe health complications like cardiovascular disease, chronic kidney disease, and even death. Existing treatments have limitations in efficacy, tolerability, and convenience. There is a significant unmet need for improved therapies that can produce robust and sustained metabolic benefits for patients.
At the core of Carmot’s drug discovery efforts is their Chemotype Evolution platform. This technology aims to rapidly navigate chemical space to identify novel small molecule drug candidates. The key steps of the platform are:
1. Identify a biological target of interest and a “bait” molecule that binds that target. The bait can be a known ligand, inhibitor, etc.
2. Derivatize the bait with reactive linkers to allow covalent bonding with fragment molecules.
3. Screen a proprietary library of fragment molecules against the target. Fragments that bind in proximity to the bait will form stable adducts.
4. Test the bait-fragment adducts for activity against the target using biochemical or biophysical assays.
5. Select the most active adducts, identify their structure, and synthesize follow-up compounds. The bait can also be altered.
6. Repeat the process using the new active compounds as baits to further optimize potency, selectivity, and drug-like properties.
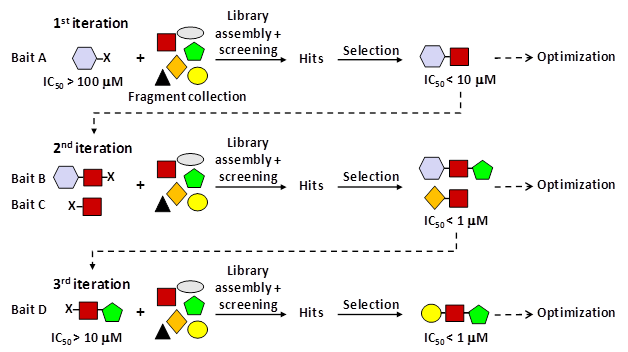
This iterative screening approach allows for the rapid exploration of vast chemical space. Rather than screening a static library, the screening library is constantly changing as new bait-fragment combinations are generated. Even a modest fragment library of 1000 compounds could generate 1000^3 (1 billion) possible adducts after just 3 cycles. This enables remarkably efficient optimization of initial hit molecules.
The Chemotype Evolution platform shares conceptual similarities with other fragment-based screening approaches like SAR by NMR. However, a key difference is that CE starts with an initial bait compound rather than a blank slate. This allows the platform to rapidly focus on chemical space relevant to the biological target of interest. Overall, CE offers an innovative combination of fragment-based screening, covalent tethering, and iterative optimization.
Carmot is using the CE platform to develop a pipeline focused on treating obesity, type 2 diabetes (T2D), and type 1 diabetes (T1D). Their lead programs target peptide hormone receptors called GLP-1 and GIP, which regulate glucose metabolism and food intake.
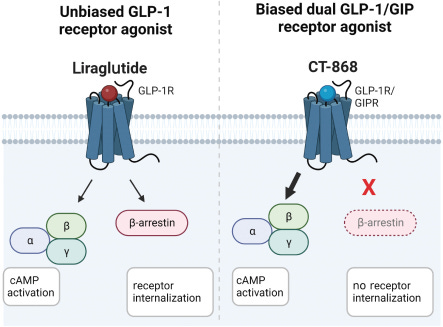
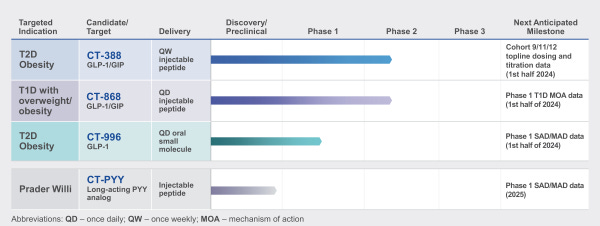
CT-388 is a long-acting GLP-1/GIP dual agonist designed for weekly subcutaneous injection. It is currently in Phase 1/2 development for obesity and T2D. In preclinical studies, CT-388 demonstrated biased signaling at both the GLP-1 and GIP receptors. This meant potent activation of beneficial cAMP signaling with minimal β-arrestin recruitment. These biased properties could lead to increased therapeutic activity and tolerability compared to unbiased agonists.
In June 2023, Carmot announced positive initial clinical data from the ongoing CT-388 Phase 1/2 trial. Statistically significant weight loss was seen across all doses after just 4 weeks of treatment. In the highest dose cohort, participants lost an average of 8.4% of their body weight. CT-388 also displayed good tolerability with mild GI-related side effects consistent with other GLP-1 therapies. These results provide clinical proof-of-concept for CT-388 and validation of the CE platform.
CT-996 is an oral small molecule GLP-1 agonist in Phase 1 development for obesity and T2D. Oral administration offers a major advantage in convenience for patients over injectable drugs. In October 2023, Carmot reported supportive preliminary Phase 1 data showing pharmacokinetics compatible with once-daily oral dosing. CT-996 was also well tolerated with mostly mild GI side effects. An efficacious oral GLP-1 therapy would be an important advance for diabetes and obesity treatment.
CT-868 is an injectable GLP-1/GIP dual agonist in development as an adjunct therapy to insulin for T1D. T1D patients have an impaired ability to produce insulin. CT-868 aims to improve glycemic control and reduce insulin needs in T1D. It is currently in Phase 2 trials. Early clinical data indicates CT-868 can lower blood glucose and HbA1c (a measure of glucose control). An adjunct therapy that reduces insulin requirements could meaningfully improve outcomes for T1D patients. In addition to these clinical programs, Carmot is also pursuing earlier stage research into new metabolic disease targets like PYY.
Carmot Therapeutics’ have to potential to play an important role in the metabolic disease space. Their innovative Chemotype Evolution platform allows for the rapid design and optimization of small molecule drug candidates. This technology has fueled a promising therapeutic pipeline, including novel GLP-1/GIP dual agonists with differentiated clinical profiles. Initial proof-of-concept data provides clinical validation of Carmot’s approach. Carmot’s patient-focused pipeline has strong potential to provide improved treatment options for patients with obesity, T2D, and T1D.
In late 2023, Carmot was acquired by Roche for $2.7B upfront plus up to $400 million in milestone payments. The acquisition gives Roche access to Carmot’s pipeline and expertise in metabolic biology to develop new therapies. The deal is expected to close in Q1 2024. The acquisition reflects Roche’s strategy to grow through targeted deals to enhance its pipeline in specific disease areas like obesity and diabetes.
Carmot Therapeutics Extends Drug Discovery Collaboration with Amgen – Press Release 2/16/2016
Carmot Therapeutics announced today it has extended the research collaboration and license agreement with Amgen Inc. (Thousand Oaks, Calif.) that was first announced in 2014. Carmot will continue to apply its proprietary lead-identification technology, Chemotype Evolution, to further advance molecules discovered during the initial collaboration. Amgen will be solely responsible for the clinical development of any molecules discovered as part of the collaboration.
Under the terms of the agreement, Carmot is entitled to fully supported research funding and pre-clinical and clinical milestone payments. In addition, a royalty will be paid to Carmot on commercial sales of products emerging from the collaboration.
“This extension of the collaboration with Amgen shows continued validation of our lead-identification technology, Chemotype Evolution,” said Carmot CEO Dr. Stig K. Hansen. “We’ve been able to identify novel chemical matter that has been validated by X-ray crystallography for two targets that historically have been extremely challenging, and we expect these molecules to be advanced in collaboration with the strong scientific team at Amgen.”
About Carmot Therapeutics, Inc.
Carmot is pioneering a transformative lead-identification approach, Chemotype Evolution, to identify superior therapeutics for human diseases. Chemotype Evolution is a proprietary technology that dramatically expands the repertoire of chemical diversity for drug discovery, providing the opportunity to tackle therapeutic targets refractory to traditional approaches. Carmot is using Chemotype Evolution to identify and optimize innovative drugs for difficult therapeutic targets, thereby addressing important unmet chemical needs.
Founded by Drs. Stig K. Hansen and Daniel A. Erlanson, Carmot has built a powerful discovery approach based on Chemotype Evolution that can rapidly and efficiently unlock novel, diverse, chemical space that is difficult to access by conventional small molecule discovery technologies. For its internal pipeline, Carmot is using Chemotype Evolution to discover superior drug candidates targeting validated pathways in metabolic disease, oncology and inflammation, with advanced candidates in metabolic disease entering IND enabling studies in 2016.
Carmot Therapeutics와 Amgen의 공동연구는 매우 성공적이어서 Amgen의 Lumakras (Sotorasib, AMG510)의 FDA 승인으로 현재 상용화되었습니다. 먼저 ACS Med Chem Lett 2019년에 Carmot Therapeutics의 Chemotype Evolution에 대한 부분이 있습니다.
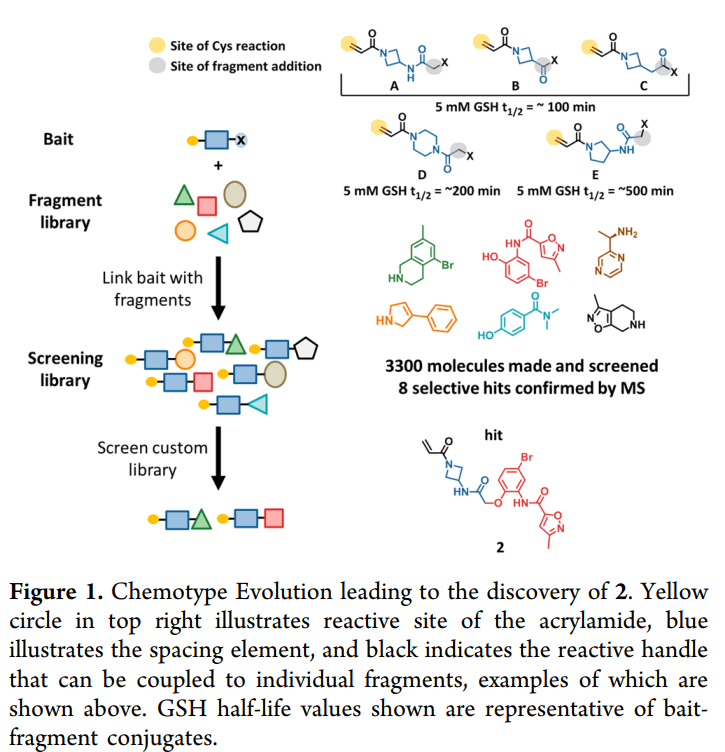
Carmot Therapeutics의 Chemotype Evolution 및 Lead Optimization을 통해 Amgen은 결국 Lumakras (Sotorasib, AMG510)을 발굴하게 되었고 그 스토리는 Journal of Medicinal Chemistry에 2020년에 발표하였습니다.
Amgen의 Lumakras (Sotorasib, AMG510) 개발부터 승인까지의 스토리는 2021년 Drugs에 리뷰가 되었습니다. – Lumakras (Sotorasib) – Amgen Website
Amgen과 연구계약을 한 후 Roche/Genentech과 Dual GLP-1R/GIP-R Agonist 개발에 대한 공동연구계약을 발표했습니다.
Carmot Therapeutics, Inc. Enters Into Discovery Collaboration With Genentech – Biospace 7/6/2016
Carmot Therapeutics announced today that it has entered into a drug discovery collaboration and license agreement with Genentech, a member of the Roche Group. During the collaboration, Carmot will apply its proprietary lead-identification technology, Chemotype Evolution, to discover novel drug hits. Carmot and Genentech will work together to identify lead candidates, while Genentech will be solely responsible for lead optimization, pre-clinical and clinical development, manufacturing, and commercialization activities.
Under the terms of the agreement, Carmot will receive an undisclosed upfront payment and is eligible to receive milestone payments based on achievement of certain predetermined pre-clinical and clinical milestones. In addition, Carmot is eligible to receive royalties on sales of certain products resulting from the license agreement. Financial terms have not been disclosed.
“Signing this new discovery collaboration with Genentech is an important step as we continue to build additional value in the company through strategic partnerships around our proprietary chemistry platform, Chemotype Evolution. We look forward to closely working with scientific teams at Genentech to deliver potent new lead compounds for their programs,” said Carmot CEO, Dr. Stig K. Hansen.
About Carmot Therapeutics, Inc.
Carmot Therapeuticsis pioneering the discovery and development of innovative drugs for the treatment of metabolic diseases, cancer, and inflammation. Carmot’s vision is to become a leader in drug discovery by generating superior drugs for challenging therapeutic targets. Chemotype Evolution, Carmot’s proprietary technology, enables the rapid identification of novel drugs through an evolutionary discovery paradigm and has produced a pipeline of breakthrough therapeutics currently in pre-clinical development. Over the past few years, Carmot has built Chemotype Evolution into a robust technology that has yielded novel lead compounds targeting incretin receptors (GLP-1R and GIP-R) for the treatment of Type 2 diabetes, obesity, and NASH and protein-protein interactions (NEMO/IKK) for the treatment of cancer and inflammation. Carmot plans to enter Phase 1 clinical testing in 2017 with a novel, differentiated dual GLP-1/GIP receptor agonist.
CT-388 (long-acting GLP-1/GIP dual agonist designed for weekly subcutaneous injection)에 대한 포스터를 2023년 Obesity Week Annual Meeting에서 발표했습니다.
CT-868 (Dual GLP-1R/GIP Modulator)에 대한 Preclinical 결과는 ADA 2023년에 발표한 포스터가 있습니다.
CT-868의 임상2상 결과는 2023년 Obesity Week Annual Meeting에서 포스터로 발표했습니다.
Carmot Therapeutics Announces Close of Series B Financing – Press Release 1/17/2018
Carmot Therapeutics, Inc. (Berkeley, CA), a biotechnology company dedicated to the discovery and development of innovative therapeutics generated through Chemotype Evolution, announced today that it has closed a $15M financing. The funds will support development of the company’s lead type 2 diabetes drug, a dual GLP-1R/GIPR agonist, through early clinical proof of concept.
The round was co-led by new investor Horizons Ventures of Hong Kong and existing investor The Column Group, and joined by private investors including Jerome Dahan. Patrick Zhang of Horizons Ventures has joined the board of directors as an observer, and Adriana Tajonar, PhD, from The Column Group has assumed the director position held by Larry Lasky, PhD, who joined Carmot’s scientific advisory board.
“We are thrilled to have secured financing for the advancement of our transformative diabetes program through clinical proof of concept”, said Dr. Stig K. Hansen, Carmot’s CEO. “We are delighted to expand our team of dedicated and visionary investors and welcome Patrick Zhang and Adriana Tajonar to the board. Together with non-dilutive revenue, this financing allows us to progress several pre-clinical leads in the areas of diabetes, obesity and fatty liver disease. The funds will also allow expansion of our efforts targeting de-ubiquitinating enzymes.”
Carmot recently relocated its headquarters to a new facility in Berkeley, CA, while maintaining a research site in Mission Bay, San Francisco. The company has ongoing collaborations with leading pharmaceutical companies, including Amgen and Genentech, to use Chemotype Evolution to identify novel drugs in multiple therapeutic areas.
About Carmot Therapeutics, Inc.
Carmot Therapeutics (“Carmot”) is a biotechnology company dedicated to the discovery and development of innovative medicines for a variety of clinical indications. Carmot applies a transformative drug discovery approach, Chemotype Evolution, to identify superior therapeutics internally and in collaboration with industry partners. Chemotype Evolution is a proprietary technology that overcomes major limitations in existing drug discovery approaches, providing Carmot a unique opportunity to tackle challenging disease targets. Carmot has identified drug leads targeting class-B GPCRs, protein-protein interactions, and de-ubiquitinating enzymes and is advancing a portfolio of wholly-owned programs in metabolic disease and oncology toward clinical development.
Carmot Therapeutics, Inc. (Berkeley, CA), a biotechnology company focused on bringing transformative therapies to patients with metabolic diseases, announced today a $47 million series C financing. The funding will support initiation early in 2021 of a 26-week dose ranging phase 2 study for CT-868 and phase 1-2 studies for CT-388 and involve more than 300 patients. Both programs are dual modulators of the GLP- 1 and GIP incretin receptors and have the potential to be best in a new class of treatments for type 2 diabetes, obese and fatty liver disease patients.
“Leveraging our transformative drug discovery platform, Chemotype Evolution (CE), Carmot has developed deep expertise in therapeutics targeting the GLP-1 and GIP incretin receptors,” commented Stig K. Hansen, PhD, Carmot’s co-founder and Chief Executive Officer. “Based on CT-868’s unique pharmacology we have seen a potential best in class therapeutic window in phase 1 studies that could translate into unprecedented improvements in HbA1c and weight loss for diabetic patients. In addition, both CT-868 and CT-388 have shown pre-clinically to be powerful insulin sensitizers which could translate into profound clinical benefits for patient populations across metabolic diseases”.
Amgen joined existing investors, The Column Group and Horizons Ventures, and other institutional investors in the round. In conjunction with the financing, Peter Svennilson, founder and managing partner of The Column Group, joined Carmot’s Board of Directors. In addition, James Watson joined Carmot as Chief Business Officer and led the series C financing process. Previously he was CBO & President ICT at Sigilon Therapeutics where he led a $485m diabetes partnership with Lilly. Prior, Mr. Watson was CEO of a boutique, life science investment bank and held leadership roles with Alvine, Incyte and Eli Lilly.
On joining the Board, Peter Svennilson commented: “Carmot has already developed valuable clinical assets, an innovative pipeline and a proven drug discovery platform. In addition, the Carmot team has a track record of successful strategic partnerships including the discovery collaboration with Amgen that led to Amgen’s breakthrough new KRAS inhibitor AMG 510. The investors and leadership team look forward to important new clinical data and attractive opportunities for further strategic partnerships and financing, all in the next 18 months”.
About Carmot Therapeutics, Inc.
Carmot Therapeutics (“Carmot”) is focused on the discovery and development of transformative therapies for patients with metabolic disease. Carmot applies Chemotype Evolution (CE), a pioneering drug discovery technology, in combination with unique biological expertise to identify innovative and superior therapeutics. In metabolic disease, Carmot is combining CE with novel insights into incretin receptor signaling to develop a broad, valuable pipeline of peptide-based and small molecule therapeutics. Carmot’s lead program, a dual GLP-1/GIP receptor modulator, is entering phase 2 development and has the potential to be best in a new class of treatment for type 2 diabetes, obese and fatty liver disease patients. In addition, Carmot is internally, and with partners, using CE to identify novel covalent inhibitors and to develop new therapeutics targeting major oncogenic pathways. Carmot has successfully applied CE with strategic partners including the discovery collaboration with Amgen that led to AMG 510, the first KRAS inhibitor to enter the clinic.
금년 1월말에 Carmot Therapeutics는 Roche에 인수를 완료했습니다. Carmot Therapeutics의 기술인 Chemotype Evolution 기술은 지난 14년간 환자들에게 유용한 신약을 만들어낼 수 있슴을 증명했습니다. Amgen의 Lumakras의 성공에 이어 Roche/Genentech에서도 비만치료제 분야에서 성공해서 환자들에게 많은 도움을 주기를 바랍니다.
Carmot Therapeutics Announces Completion of Acquisition by Roche – Biospace 1/29/2024
BERKELEY, Calif., Jan. 29, 2024 (GLOBE NEWSWIRE) —Carmot Therapeutics, Inc. (Carmot), a clinical-stage biotechnology company dedicated to developing life-changing therapeutics for people living with metabolic diseases including obesity and diabetes, today announced that its acquisition by the Roche Group (Roche) has been completed.
Having successfully completed its acquisition of Carmot, Roche obtains access to Carmot’s current R&D portfolio including all clinical and pre-clinical assets, as well as exclusive access to Carmot’s innovative Chemotype Evolution discovery platform in metabolism, further strengthening Roche’s R&D efforts and portfolio across cardiovascular and metabolic diseases. Carmot and its employees will join the Roche Group as part of Roche’s Pharmaceuticals Division.
The acquisition gives Roche access to Carmot’s differentiated portfolio of incretins including:
- CT-388, the lead asset, is a Phase-2 ready, dual GLP-1/GIP receptor agonist for the treatment of obesity in patients with and without type 2 diabetes. Injected subcutaneously once a week, it has potential as a standalone and combination therapy to improve weight loss and to be expanded to other indications.
- CT-996, a once-daily oral, small molecule GLP-1 receptor agonist currently in Phase-1 intended to treat obesity in patients with and without type 2 diabetes.
- CT-868, a Phase-2, once-daily subcutaneous injectable, dual GLP-1/GIP receptor agonist intended for the treatment of type 1 diabetes patients with overweight or obesity.
Financial Considerations
Roche has acquired all outstanding shares and options of Carmot at a purchase price of $2.7 billion. Carmot’s equity holders are additionally entitled to receive payments of up to $400 million depending on the achievement of certain milestones.
About Obesity
Obesity is one of the most pervasive health challenges in the world and an area where recent scientific advances can help meet the high unmet medical need. This condition is associated with many health challenges and comorbidities, including type 2 diabetes, cardiovascular diseases, fatty liver, and chronic kidney disease, which together place an incredible strain on healthcare systems worldwide. Over 4 billion people are estimated to be obese or overweight by 2035, approaching 50% of the world’s population.1
Scientific advances in the field of incretins and an increased understanding of relevant disease biology have significantly changed the possibilities to treat obesity over the last years. Incretins are gut hormones that are secreted after food intake and play a role in modulating blood glucose by stimulating insulin secretion. Emerging scientific data show a wider biologic effect of incretins in multiple organs including the liver, heart and brain, suggesting they may have broader roles in the body. Incretins are clinically validated targets and the emerging standard of care therapies in obesity and could also be effective targets in other disease areas.
About Carmot Therapeutics
Carmot Therapeutics is a clinical-stage biotechnology company dedicated to developing life-changing therapeutics for people living with metabolic diseases, including obesity and diabetes. Carmot’s expertise in metabolic biology has enabled the development of a broad pipeline of therapeutics, including three clinical candidates: CT-388 (once-weekly subcutaneous injectable, dual GLP-1/GIP receptor agonist), CT-996 (once-daily oral, small molecule GLP-1 receptor agonist) and CT-868 (once-daily subcutaneous injectable, dual GLP-1/GIP receptor agonist) and other molecules in preclinical development. All of these are proprietary novel compounds, wholly owned by Carmot, that have the potential to deliver an enhanced treatment response in people with metabolic diseases. For more information, visit the Carmot Therapeutics website.
References
1 World Obesity Atlas 2023, https://data.worldobesity.org/publications/WOF-Obesity-Atlas-V5.pdf
