(Picture: Daniel K. Nomura, University of California at Berkeley & Co-Founder of Frontier Medicines)
안녕하세요 보스턴 임박사입니다.
UC Berkeley의 Daniel K. Nomura교수팀은 Chemoproteomics Platforms을 이용하여 Covalent Drug Discovery 를 할 수 있다는 연구결과를 발표했고 이를 기반으로 Frontier Medicines가 설립되었습니다.
Frontier Medicines Closes $67 Million Series A, Will Focus on Chemoproteomics – Biospace 6/25/2019
Frontier Medicines closed on a Series A financing round worth $67 million. The round was led by Deerfield Management, DROIA Oncology Ventures and MPM Capital, with participation from DCVC Bio, RA Capital Management and other investors.
The company will focus on chemoproteomics, which it calls a way of interrogating proteins in living systems. This allows them to identify potential new binding targets on proteins, which will allow the development of small-molecule drugs. The company’s technology platform integrates advanced computation and machine learning.
“Our platform currently includes a database of hotspots that cover a majority of human proteins, including those that were previously considered ‘undruggable;’ an expanding library of diverse, covalent compounds being driven by machine learning; and a novel approach to protein degradation,” stated Daniel K. Nomura, co-founder of Frontier. “This platform enables us to go after almost any protein target of interest for therapeutic intervention.”
The company was founded by Nomura, an associate professor of Molecular and Cell Biology, Chemistry, Nutritional Science and Toxicology at UC Berkeley and Chris Varma, who will act as chief executive officer and president. Varma is a well-known entrepreneur who co-founded and was the former chief executive officer of Blueprint Medicines. And finally, the third co-founder is Roberto Zoncu, assistant professor of Molecular and Cell Biology at UC Berkeley.
“Our therapeutic programs are focused on several of the most important and difficult targets in cancer,” stated Varma. “With our platform, we have the ability to address previously inaccessible disease-causing proteins. While we are taking on a considerable challenge, we believe this approach will have a tremendous impact on transforming patients’ lives for the better, which is our ultimate goal.”
The company’s board of directors will include Varma, who is joined by Luke Evnin of MPM Capital, Othman Laraki, an independent board member, Janwillem Naesens of DROIA Oncology Ventures, and Cameron Wheeler of Deerfield Management.
Johannes Hermann is Frontier’s chief technology officer. He was previously the global Head for Data Science at Johnson & Johnson Medical Devices Technology. Before that, he was the head of the Machine Learning and Advanced Analytics department at Janssen Pharmaceuticals, a J&J company.
Frontier Medicines에는 Carmot Therapeutics에서 Amgen의 KRAS G12C Covalent Inhibitor인 Lumakras를 개발한 Daniel Erlanson 박사가 Chief Innovation Officer로 연구를 주도하고 있습니다. Erlanson 박사는 Chemotype Evolution이라는 Fragment-based drug discovery (FBDD) 분야의 리더입니다.

(Picture: Daniel Erlanson, PhD, CIO at Frontier Medicines)
2020년에 Abbvie와 공동연구계약을 체결했고 $55 Million upfront와 $45 Million Milestones 를 포함 총 규모 $1 Billion 계약입니다. E3 Ligase Small molecule을 개발하는 목적을 가지고 있습니다.
AbbVie pays Frontier $55M to pursue hard-to-drug targets – Fierce Biotech12/3/2020
AbbVie has teamed up with Frontier Medicines to develop molecules against hard-to-drug targets. The collaborators will use Frontier’s technology for uncovering hidden binding pockets to develop protein degraders and small molecules against oncology and immunology targets.
Frontier, a 2019 Fierce 15 company, is built on a platform designed to spot temporary binding sites that open up when proteins move. The platform could reveal ways to drug targets that have long been recognized as therapeutically important but have remained inaccessible to researchers due to their lack of obvious binding sites.
AbbVie has bought into the idea. The Big Pharma is paying $55 million to enter into a multiyear R&D collaboration with Frontier. AbbVie is also on the hook for up to $45 million in milestones over the next 12 months, plus R&D costs and a package of success-based payments that could top $1 billion.
The commitments have landed AbbVie the chance to work with Frontier in several areas relevant to its target-discovery technology. AbbVie has tasked Frontier with discovering small molecules directed to E3 ligases, the enzymes that drive the ubiquitination and degradation of proteins. That concept is at the heart of the work of biotechs including Arvinas and Kymera Therapeutics.
AbbVie moved into the protein degradation space in 2019 through a pact with Mission Therapeutics in Alzheimer’s and Parkinson’s diseases. The Frontier collaboration opens another front in AbbVie’s work on protein degradation while also giving it a chance to go after oncology and immunology targets.
In disclosing the collaboration, AbbVie said it has selected certain targets that “are considered well validated but to date, inaccessible.” Frontier’s technology could render the targets accessible and, in doing so, position AbbVie to act on validated biology for the first time.
AbbVie and Frontier will collaborate on the research and preclinical aspects of the programs. Once a project passes “defined stages of preclinical development,” AbbVie will take over and handle global development and commercialization. The deal gives Frontier the option to share the costs and work for some oncology programs up to the end of phase 2. AbbVie has an option on additional targets.
The relationship provides external validation to Frontier’s approach. Frontier broke cover last year with a $67 million series A led by Deerfield Management, Droia Oncology Ventures and MPM Capital. Helmed by former Blueprint Medicines CEO Chris Varma and armed with technology developed at the lab of the University of California, Berkeley’s Daniel Nomura, Frontier immediately established itself as a notable name in the expanding pool of biotechs going after “undruggable” targets but lacked a Big Pharma partner.
Frontier Medicines Raises $88.5 Million to Advance Chemoproteomics Program – Biospace 7/19/2021
Frontier Medicines launched in 2019 with $67 million in financing and a focus on chemoproteomics, which the company described as a method of interrogating proteins in a living system. This is expected to target new spots on cancer cell proteins the company calls hotspots.
A significant amount of protein surface cannot be targeted by small molecules due to the lack of binding sites. However, the idea of chemoproteomics follows the reasoning that as proteins move within the cells, they created temporary hotspots that new drugs can target. So far, Bar Area-based Frontier Medicines has identified more than 150,000 hotspots on proteins of interest that have the potential to expand the company’s pipeline.
Last year the company partnered with AbbVie to develop small-molecule therapeutics against high-interest protein targets, including the approximately 600 E3 ligases.
“Between the substantial protein degradation partnership with AbbVie announced at the end of last year and this financing round, we have significantly strengthened our resources to deliver on our vision of developing breakthrough medicines for patients,” Chris Varma, chairman, chief executive officer, and co-founder of Frontier Medicines said in a statement.
The addition of the $88.5 million from the Series B round will be used to advance this focus. Frontier’s lead inhibitor is aimed at both activated and inactivated forms if KRASG12C, which has been linked to different cancer types, including non-small cell lung cancer, colorectal carcinoma, and pancreatic ductal adenocarcinoma.
Frank McCormick, a professor at the UCSF Helen Diller Family Comprehensive Cancer Center specializing in KRAS biology and a member of Frontier Medicines Scientific Advisory Board, said the ability to target both the active and inactive states of KRASG12C with a small molecule therapeutic is a “long-awaited scientific breakthrough.” A medication with this dual form of inhibition is likely to be more efficacious than a drug that targets the inactive form of the protein only. McCormick said a dual inhibitor could address the “large majority of patients who are non-responders to first generation single-form KRASG12C inhibitors, as well as those patients whose tumors become resistant to the first-generation molecules.”
The Series B financing round was co-led by Woodline Partners LP and RA Capital Management. Deerfield Management Company had equal participation in the round. New investors in the Series B included Deep Track Capital, ArrowMark Partners, Driehaus Capital Management, and Sphera Healthcare. Existing investors DCVC, Droia Ventures and MPM Capital also participated in the Series B financing round.
In addition to the continued development of its pipeline, Frontier Medicines will use some of the Series B funds to expand into the Boston market. The company will open what it called a “state-of-the-art facility” focused on research and development and discovery, pre-clinical development, translational medicine, and early clinical development.
The Bay State facility will be integrated with Frontier’s Bay Area headquarters. In its announcement, Frontier Medicines did not provide information on how many people will be working out of its planned Boston site.
Frontier Medicines also formed a Scientific Advisory Board to help guide its research. The board includes Joan S. Brugge, director of the Harvard Ludwig Cancer Center; Giulo Draetta, the chief scientific officer at the University of Texas MD Anderson Cancer Center; Steven Gygi, a professor of Cell Biology at Harvard Medical School; William C. Hahn, the William Rosenberg Professor of Medicine and chief operating officer of Dana-Farber Cancer Institute; Kevin Koch, the former CSO of Array BioPharma and a venture partner with OrbiMed; Frank McCormick, a professor at the UCSF Helen Diller Family Comprehensive Cancer Center and an expert in KRAS biology; Daniel K. Nomura, co-founder of Frontier Medicines and leading expert in chemoproteomics; and Roberto Zoncu, a co-founder of Frontier Medicines and an expert in cancer biology, small GTPase signaling, and autophagy.
Frontier Medicines Raises $80M in Series C, Targets Amgen and BMS in KRAS – Biospace 2/23/2024
Frontier Medicines on Thursday closed its oversubscribed $80 million Series C funding round, which it will use to advance its potentially first-in-class next-generation KRAS blocker FMC-376.
The financing brings Frontier’s total capital raised to $235.5 million since its founding, according to the announcement. The funding round was co-led by Deerfield Management Company and Droia Ventures. Galapagos NV, a Belgium-based pharmaceutical company, participated as a strategic investor, along with contributions from MPM Capital, RA Capital Management and DCVC Bio.
Frontier also announced on Thursday that it had dosed the first patient in the Phase I/II PROSPER trial, testing FMC-376 in patients with G12C-mutated KRAS cancers.
CEO Chris Varma in a statement called the development a major milestone for the company. “Frontier Medicines has amassed a robust data set that shows FMC-376 is expected to overcome the resistance seen with prior generating single-acting inhibitors, and we are excited to demonstrate this potential in the clinical setting.”
FMC-376 distinguishes itself from other KRAS inhibitors by directly engaging both the inactive and active forms of the G12C-mutated KRAS. This differentiated dual direct mechanism of action could help FMC-376 to potentially overcome the treatment resistance and suboptimal response observed in single-acting KRAS inhibitors.
In lab studies, FMC-376 showed activity against a wide range of KRAS G12C mutant cancer models, including non-small cell lung cancer (NSCLC), pancreatic cancer and colorectal cancer.
Frontier presented preclinical data for the candidate in April 2023 at the annual meeting of the American Association for Cancer Research, demonstrating that FMC-376 is more than 1,000-fold more effective at blocking the interactions of key effector proteins compared with prior-generation inhibitors. This blocking mechanism results in a “rapid and durable” inhibition of KRAS G12C signaling, according to the company.
With FMC-376, Frontier is looking to challenge Amgen and the recently BMS-acquired Mirati in the KRAS arena.
Amgen’s Lumakras (sotorasib) won the FDA’s accelerated approval in May 2021 for the treatment of NSCLC patients carrying the KRAS G12C mutation. To keep it on the market, Amgen ran the Phase III CodeBreaK 200 study as a confirmatory trial to verify Lumakras’ clinical benefit.
However, in October 2023, the FDA’s Oncologic Drugs Advisory Committee found that progression-free survival data from the study could not be reliably interpreted and voted 10–2 against Amgen. The FDA agreed with the advisory committee two months later, denying full approval for Lumakras and requesting an additional confirmatory trial due no later than February 2028.
Mirati’s Krazati (adagrasib) was granted accelerated approval in December 2022 for the same indication. The KRAS blocker then secured a positive opinion from the European Medicine Agency’s Committee for Medicinal Products for Human Use in November 2023. BMS bought Mirati for $4.8 billion in October 2023, with Krazati as one of the acquisition’s prized assets.
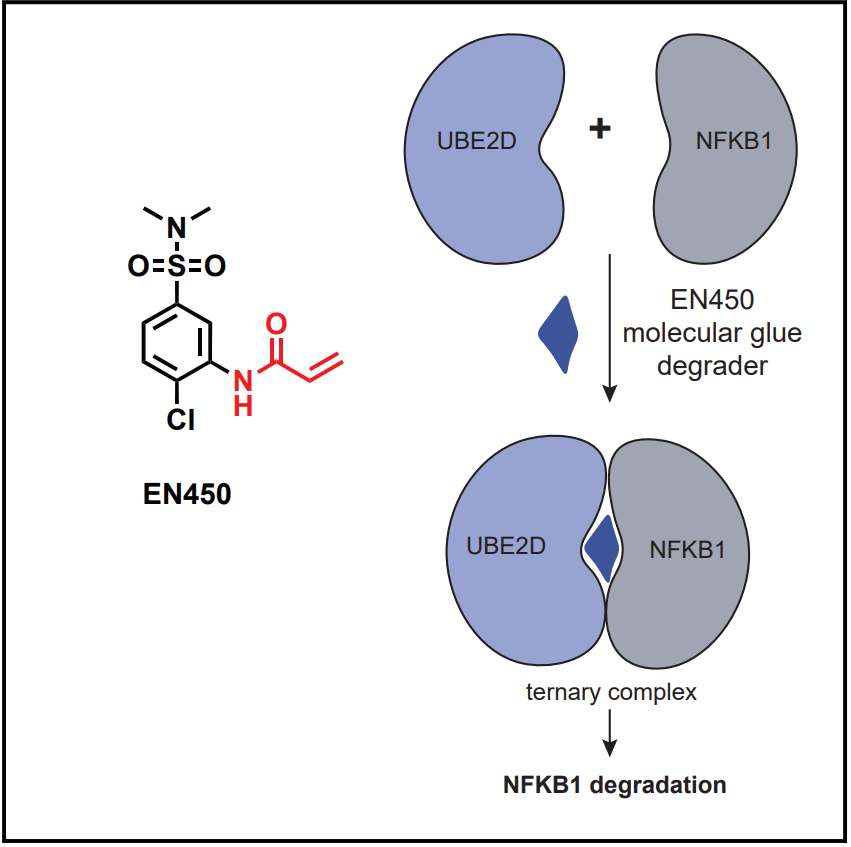
Frontier의 공동창업자인 Daniel Nomura 교수팀은 2023년 Cell Chemical Biology에 Covalent Molecular Glue Degrader 발견에 대해 보고했습니다. 현재 KRAS G12C 약물은 Amgen의 Lumakras와 함께 BMS/Mirati의 Krazati (Adagrasib)이 FDA 승인을 받은 바 있습니다.
BIOTECH (6) – Mirati Therapeutics
Lumakras 개발자가 CIO로 참여하고 있고 Krazati 개발 CSO가 Board of Directors로 참여하고 있는데 과연 FMC-376이 이 두 약물을 넘어설 수 있을지 기대가 되는군요.