(Picture: Thomas Thum, MD, PhD, Professor of Hanover Medical School, Co-Founder, CSO, CMO of Cardior Pharmaceuticals)
안녕하세요 보스턴 임박사입니다.
micro RNA (miRNA)가 다시 돌아왔습니다. Antisense Oligonucleotide, siRNA와 함께 주목을 받았지만 miRNA는 임상에서 성공하지 못하고 속수무책으로 미국의 miRNA 바이오텍이 스러져 갔습니다. 그런데 이런 와중에도 독일 하노버에 있는 Cardior Pharmaceuticals GmbH는 2016년에 설립된 이래 꾸준히 연구를 지속해서 마침내 임상2상까지 좋은 약물을 만들어낸 것으로 인정되어 오늘 Novo Nordisk에 인수된다는 발표가 났습니다. 정말 꿈인지 생시인지 알 수가 없군요.
Cardior Pharmceuticals는 Hanover Medical School 교수인 Thomas Thum 박사의 오랜 miRNA 연구결과를 바탕으로 CEO인 Claudia Ulbrich박사와 공동으로 2016년에 창업되었습니다.

Thomas 교수는 mir-212와 mir-132가 항암제인 Doxorubicin으로 인한 Atrophy와 Cardiotoxicity를 줄인다고 보고한 연구결과를 Molecular Therapy 2019년에 발표했습니다.

Cardior의 접근법은 두가지 점에서 기존의 miRNA 회사들과 차이가 있습니다.
- Oligonucleotide Chemistry에서 LNA (Locked Nucleic Acid)를 사용해서 효과와 안전성을 높였습니다.
- 200여마리의 돼지 (Porcine) 동물모델을 이용해서 임상시험을 지지할 훌륭한 전임상 데이타를 확보했습니다.
PCT 2020/249713 Al에 따르면 CDR132L의 구조는 아래와 같습니다. 5’부터 염기 하나씩 건너서 LNA가 있고 Phosphorothioate linkage로 연결되어 있는 16-mer Oligonucleotide입니다.

Thomas Thum 교수의 연구실적에 대해서는 Fraunhofer 연구소에서 2021년에 게재한 바가 있는데 Cardiology와 RNA Biology 분야의 탄탄한 연구업적이 있는 과학자라고 소개가 되었습니다.
For the second time already, Prof. Dr. Dr. med. Thomas Thum is one of the most frequently cited scientists worldwide. Once a year, the U.S. company Clarivate Analytics reports which publications in a particular discipline are among the one percent that have been cited most. Thomas Thum is a specialist in cardiology and bioscience, with a clear research focus on the functional characterization and translational potential of therapeutic RNA strategies, and his publications have had a major impact on the progress of cardiac research.
Citations are the most important currency in the world of science. Results published by scientists in peer-reviewed journals can be used by other researchers for their own work, as long as they are clearly indicated as citations. The number of citations, however, is not the only indicator of the impact of a scientist: the reputation of the journal in which an article is published also plays an important role – and so it does in the evaluation criteria applied by Clarivate Analytics.
Thomas Thum is one of the most important pioneers of RNA-based therapeutics. With his team at the Hannover Medical School he was the first to successfully test an RNA compound for the treatment of heart failure patients in a clinical trial. He joined the Fraunhofer Institute for Toxicology and Experimental Medicine ITEM in Hannover (Germany) as institute director on January 1, 2021 and has since been managing this institute together with Prof. Norbert Krug. “To be a part of the global Who’s Who of science is really a great honor for me and makes me very happy,” Thum commented on this success. “I am quite optimistic that patients will soon be able to benefit from the findings of our research in clinical practice.” Prof. Thum is listed in the “Cross Fields” category, indicating that his work has an impact on science beyond his actual field of research.
For a complete list of the most highly cited researchers, please refer to the Internet page of Web of Science: https://recognition.webofscience.com/awards/highly-cited/2021/
Cardior Pharmaceuticals는 설립 후 1년이 지난 2017년에 EUR 15 Million ($17 Million) Series A를 했습니다. 이 때 Boehringer Ingelheim과 BMS가 참여했습니다.
Cardior Pharmaceuticals raises €15 million in series A financing – Bionty 5/11/2017
Cardior Pharmaceuticals, a spin-off from Hannover Medical School (MHH), today announced the completion of a €15 million Series A financing round led by LSP (Life Sciences Partners), Boehringer Ingelheim Venture Fund (BIVF), Bristol-Myers Squibb (BMS), BioMedPartners (with its new BioMedInvest III Fund) and High-Tech Gründerfonds (HTGF). Cardior is pioneering its proprietary RNA technology to revolutionize predicting and treating heart failure. The molecular targets are non-coding RNAs linked to heart failure development that simultaneously control cardiac growth and calcium handling/contractility of cardiomyocytes. The targeting of certain specific non-coding RNAs reverses maladaptive cardiac remodeling and restores normal cardiac function.
“It is a rare opportunity to develop cutting-edge science in the area of cardiovascular diseases with a high unmet medical need. I am delighted to join Cardior at this exciting development stage of the company and together with its motivated team, quickly put on the map a novel class of drugs and companion diagnostics with the potential to prevent and overcome heart failure” said Dr. Claudia Ulbrich, Chief Executive Officer of Cardior.
“The significant funding raised at this stage of development of the company will provide the resources for an ambitious development plan for our lead compound,” added Prof. Thomas Thum, who is joining the management team as Chief Scientific Officer.
“We are very excited to be leading this financing” said Dr. Joachim Rothe, Managing Partner at LSP and a Director of Cardior. “There has been a painful lack of scientific and clinical progress in the cardiovascular field for the past 15 years, and Cardior is well positioned to change this.”
2019년에 BioWorld에서 Cardior에 대해 기사가 나온 바가 있습니다. 이 때 이미 200마리의 돼지 동물실험으로 얻은 전임상 결과를 얻은 것으로 얘기를 하고 있습니다.
2020년 Nature Communications에 Anti-miR-132 Oligonucleotide인 CDR132L의 전임상 결과를 발표했습니다. 200마리나 되는 돼지를 대상으로 광범위한 동물실험 결과를 얻었습니다. 돼지는 인간과 심장의 크기 등이 유사해서 Gold Standard로 하는 동물모델입니다.
그리고 2021년 Journal of American College of Cardiology에 돼지 동물 모델을 통해서 CDR132L이 Myocardial Hypertrophy를 감소시킨다는 사실을 증명했습니다.
2021년에는 Biocentury에서 Cardior에 대한 기사가 나왔습니다. 이 때는 CDR132L의 임상1상을 끝내고 임상 2상을 준비한다고 얘기를 했습니다.
같은해에 $76 Million Series B를 했습니다. 이 펀딩으로 CDR132L의 임상2상을 수행할 수 있고 새로운 후속 파이프라인을 개발한다는 계획이었습니다. 유럽에서 이 정도 규모의 펀딩은 흔치 않은 규모입니다.
HANOVER, Germany–(BUSINESS WIRE)–Cardior Pharmaceuticals, a clinical-stage biotech company developing non-coding RNA (ncRNA)-based therapeutics for patients with cardiac diseases, announced today the closing of a €64 million ($76 million) Series B financing round. The round was led by Inkef Capital, supported by fellow new investors Fund+, Sunstone, Hadean Ventures and Coparion with participation from existing investors including LSP, BioMedPartners, Bristol Myers Squibb and High-Tech Gründerfonds.
“We believe ncRNAs can fundamentally change the treatment of heart disease by preventing, repairing and reversing damage to cardiac tissue. We thank our new and existing investors for their support and their confidence in our ability to achieve our goal,” said Dr. Claudia Ulbrich, Chief Executive Officer and Co-Founder of Cardior. “This substantial funding, provided by leading biotech investors, validates the strength of our RNA approach and our team. We welcome our new directors and look forward to working closely with our board as we continue our rapid progress toward the start of the Phase 2 trial with our lead program CDR132L, which has the potential to demonstrate clinical proof-of-concept as a transformative heart disease treatment and to set the stage for the emergence of a new class of medicines.”
In conjunction with the financing, representatives from Inkef Capital, Fund+ and Sunstone will join the Company’s Advisory Board. The full composition of the Board can be found under the following link.
The Series B proceeds will be used to fund the late-stage clinical development of Cardior’s lead program and the expansion of the company’s earlier-stage pipeline. Lead candidate CDR132L is an oligonucleotide-based ncRNA inhibitor targeting micro-RNA-132. micro-RNAs are endogenous molecules that function as cellular regulators and their dysregulation contributes to the development of many diseases including cardiovascular diseases. Cardior’s lead program is intended to block the abnormal cardiac levels of micro-RNA-132 in heart failure patients thereby triggering a concerted therapeutic effect against key hallmarks of heart disease including cardiac hypertrophy, fibrosis, impaired contractility and reduced vascularization. Cardior’s approach is applicable to a broad range of heart diseases as represented in its development pipeline, which addresses large cardiac indications as well as rare diseases such as hypertrophic and dilated cardiomyopathies.
“Heart diseases are the leading cause of death worldwide, causing a massive burden on patients, their families and global healthcare systems,” added Dr. Simone Botti, Junior Partner at Inkef. “Cardior’s RNA approach has shown an encouraging safety and efficacy profile in its initial clinical read-out and has the potential to provide a true disease modifying therapy to patients. We are excited to support Cardior on its continued progress advancing the first ncRNA-therapeutic towards commercialization.”
“Cardior is on an exciting trajectory which is reflected in the Series B syndicate. I look forward to working with the incoming investors as we leverage the current momentum in the RNA therapy field to position Cardior for success,” said Dr. Karin Kleinhans, Partner at LSP.
***
About CDR132L
CDR132L is a highly stable water-soluble oligonucleotide ncRNA inhibitor directed to block aberrant micro-RNA-132 levels and thereby reverses the cellular pathology and restores normal function in cardiomyocytes, contributing to improved cardiac systemic and diastolic function in patients with heart failure (HF). CDR132L has completed Phase 1b development demonstrating a favorable safety profile and beneficial cardiac effects in 28 HF patients. Cardior is currently initiating a Phase II clinical trial of the antisense drug.
About Cardior
Cardior Pharmaceuticals is a leading clinical-stage biopharmaceutical company pioneering the discovery and development of RNA-based therapeutics designed to prevent, repair and reverse diseases of the heart. Cardior’s therapeutic approach uses distinctive non-coding RNAs as an innovative platform for addressing the root causes of cardiac dysfunctions. The company aspires to bring transformative therapeutics and diagnostics to patients and thereby make a lasting impact on the treatment of cardiac diseases worldwide.
About INKEF Capital
INKEF Capital is a venture capital firm based in Amsterdam, backing promising early-stage companies in Europe. INKEF takes pride in being a patient, long-term investor with the ability to support companies through several rounds of funding. From the early stages of a technology or life science venture, INKEF Capital supports entrepreneurs building their ideas into successful international businesses. For more information, please visit: https://www.inkefcapital.com/.
About LSP
LSP is one of the largest European investment firms providing financing for life sciences and health care companies. LSP’s management has raised over €2.7 billion ($3.2 billion) and supported the growth of 300 companies since it started to invest in 1988, including signature deals such as argenx, Crucell and Neuravi. With offices in Amsterdam, Munich and Boston, LSP currently has the possibility to invest through five strategies, each having a distinctive investment scope and a dedicated team: LSP 6 invests in private early- to late-stage drug development and medical technology companies; LSP HEF 2 focuses on private late-stage medical technology companies; the LSP Dementia Fund invests in companies targeting neurodegenerative diseases; LSP Public targets public healthcare companies; and EBAC is the first healthcare SPAC to exclusively focus on European biotech. LSP is an active contributor to the global life sciences industry and the European life science eco-system by assuming for-profit and not-for-profit roles as initiators, founders and board members in various private and public bodies and organizations, for example being founder and board member of the Oncode Institute. For more information: lspvc.com.
올해 2월에 있었던 학회에서 Thomas Thum 박사는 Cardior Pharmaceuticals의 CDR132L을 중심으로 Corporate Presentation을 발표했습니다.
임상 1b에서 NT-proBNP의 레벨이 23.3%의 감소를 보였고 placebo는 반대로 0.9% 상승했습니다. HFrEF subpopulation결과는 -37.1% vs +17.7% (placebo)로 격차가 더 큽니다.

이 발표 후 한달이 지난 오늘 Novo Nordisk는 $1 Billion에 Cardior를 인수한다고 발표했습니다. 지금 Novo는 GLP-1 당뇨병 및 비만치료제가 성공해서 자금 여력이 있지요. 그동안은 당뇨병 전문제약회사였는데 이제 심장병 분야도 진출하기 위해 노력하고 있습니다. 현재 IL-6 inhibitor ziltivekimab 의 임상3상이 진행 중입니다. Novo 입장에서는 First-in-class인 CDR132L의 가능성을 본 것 같습니다.
Novo Nordisk inks $1B Cardior buyout to pump up heart failure plans – Fierce Biotech 3/25/2024
Novo Nordisk is pumping up its heart failure plans. The drugmaker, swelled by its GLP-1 windfall, has decided to buy Cardior Pharmaceuticals and its midphase prospect in a deal that could top out above 1 billion euros ($1.1 billion).
Cardior is developing an antisense oligonucleotide to inhibit a piece of non-coding RNA, miR-132, that is implicated in heart failure. Upregulation of the RNA when certain cells are stressed can lead to changes in the size and shape of the heart. Blocking elevated miR-132 could therefore prevent or reverse changes that are associated with poor prognosis in patients who have heart attacks.
The biotech raised 64 million euros from investors including Bristol Myers Squibb in 2021 and used the cash to take (PDF) its oligonucleotide, CDR132L, into a phase 2 trial the next year. Cardior has designed the 280-subject study to show CDR132L’s effect on the volume of blood in part of the heart.
Cardior is still months away from the primary completion of the study, according to ClinicalTrials.gov, but Novo Nordisk is already planning to expand development. The Danish drugmaker plans to run another phase 2 trial in chronic heart failure patients with cardiac hypertrophy, a condition that negatively affects the heart’s ability to pump blood.
CDR132L will slot into a pipeline that already features heart failure programs. Novo Nordisk reported clinical trial data on semaglutide, the active ingredient in Ozempic and Wegovy, in heart failure patients last year. The company is running a phase 3 trial of the IL-6 inhibitor ziltivekimab in a heart failure patient population and is working with Heartseed to test a cell therapy in an early-phase study.
Novo Nordisk moved to buy Cardior after identifying CDR132L as a molecule with “a distinctive mode of action” that has the “potential to become a first-in-class therapy designed to halt or partially reverse the course of disease for people living with heart failure.” Advances in the treatment of heart failure have so far largely focused on managing the symptoms. CDR132L could address the underlying causes.
The potential for CDR132L to provide long-lasting improvement in heart function led Novo Nordisk to put together an offer worth up to 1.025 billion euros for Cardior. The package includes an upfront payment and milestones, but neither party has provided a breakdown of the deal.
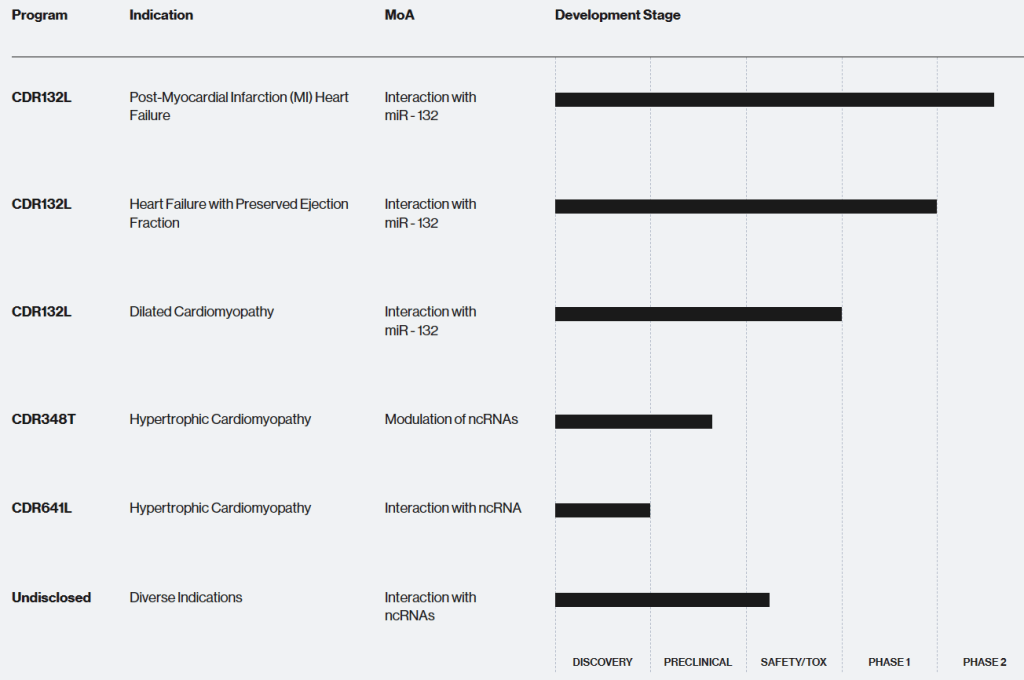
현재 홈페이지에 나타난 파이프라인은 아래와 같습니다. CDR132L은 세가지 임상이 진행 중이고 그 이외에 세개의 전임상 물질을 보유하고 있습니다. CDR132L이 승인까지 간다면 세계 최초로 승인되는 miRNA 약물이 됩니다. 정말 기대가 되는군요.