(Picture: Josep Bassaganya-Riera, Professor of Virginia Tech University, PhD, Founder of Landos Biopharma, Founder & CEO of NImmune Biopharma)
안녕하세요 보스턴 임박사입니다.
바이오텍의 성공확률이 높지 않다는 것은 놀라운 사실이 아니지만 파이프라인에 문제가 없는데도 이사회와의 갈등으로 어려움을 겪는 것은 좀 드문 경우입니다. Virginia Tech 교수이면서 LANCL2 (Lanthionine Synthetase C-Like Protein 2) 라는 표적을 발견하고 이 표적을 이용한 자가면역질환 (Autoimmune Disease) 치료제를 개발하고자 하는 노력에 대해 좀 나누고자 합니다.
Josep Bassaganya-Riera 교수는 2012년에 LANC:2 표적 약물을 Computational Discovery를 통해서 발견했다고 PLOS One에 발표하였습니다.
이 결과를 바탕으로 Landos Biopharma를 설립해서 $10 Million Series Aㄹㄹ 했습니다. Perceptive Advisors가 단독으로 참여했습니다.
Landos Biopharma Raises $10 Million in Series A Financing – Business Wire 9/21/2017
Landos Biopharma, Inc., an emerging biopharmaceutical company focused on developing improved treatments for autoimmune diseases, announced today it has raised $10 million in a Series A financing led by life sciences investment management firm Perceptive Advisors, LLC, which will serve as its exclusive investor for the Series A round.
Landos, founded by serial entrepreneur and innovator Dr. Josep Bassaganya-Riera, is advancing a robust pharmaceutical pipeline for autoimmune diseases toward commercialization and will partner operational efforts with Xontogeny, LLC, a life sciences accelerator operating company led by industry veteran Chris Garabedian. Xontogeny’s model works in synergy with partner companies to support management teams and provide expertise and strategic direction in the early stages of pharmaceutical and biotechnology development.
Current therapies for autoimmune diseases, a $100 billion market, have limited efficacy and significant side effects. Landos’ mission is to accelerate the development of safer, more effective therapeutics for painful and debilitating autoimmune diseases including Crohn’s disease and ulcerative colitis.
“I’m thrilled to have Xontogeny and Chris Garabedian as a strategic and operating partner along with Perceptive Advisors as Landos’ exclusive financier in the Series A, to bring our lead product, BT-11, through initial clinical studies in 2018,” said Landos Chairman and CEO, Dr. Josep Bassaganya-Riera “We have a committed leadership team with industry experience focused on developing oral treatments that address an unmet clinical need of patients, can disrupt the $9.2 billion per year inflammatory bowel disease (IBD) therapeutics market, and most importantly improve the lives of millions of patients living with these diseases.”
BT-11은 Omilancor라고 불리는 물질로서 발표한 자료는 아래에 링크합니다.
Landos will focus its development efforts in a novel target pathway called Lanthionine Synthetase C-Like 2 (LANCL2), which has shown promise for the treatment of autoimmune diseases by engaging a unique mechanism of action that exerts potent anti-inflammatory effects. Landos’ lead product candidate BT-11 is a first-in-class, orally active, locally-acting therapeutic for treatment of Crohn’s disease and ulcerative colitis that has shown a benign safety profile in animal models and that is advancing toward an Investigational New Drug (IND) filing. BT-11 intercepts IBD by decreasing the production of inflammatory mediators and increasing anti-inflammatory molecules within the gastrointestinal tract. IBD impacts approximately 1.6 million Americans and 4 million people worldwide.
Scientific Reports 에도 BT11에 대한 발표를 했습니다.
“Landos is an exciting company for Xontogeny to partner with as Josep and his team have produced very compelling data in animal models of IBD and have brought their lead compound, BT-11, to the cusp of clinical trials,” said Mr. Garabedian, who will serve as a Landos Board member and senior business advisor. “The properties of BT-11 are unique and well differentiated from current IBD products and we believe it has the potential to represent an advance in the treatment of both Crohn’s disease and ulcerative colitis.”
“We are extremely excited to partner with both Josep and Chris to build Landos into a global biopharmaceutical company focused on autoimmune diseases, and are convinced that the team will provide an accelerated path to compelling clinical proof-of concept for IBD,” said Joe Edelman, CEO of Perceptive Advisors. “As the first investment in a Xontogeny portfolio company since partnering with Chris earlier this year, we believe that Landos exemplifies the company profile we aim to support – an outstanding leadership team, a lead product with a novel mechanism, strong IP, and a compelling preclinical and translational dataset that is close to the clinic for the treatment of an important disease with an unmet need for safer and more effective drugs.”
With the Series A investment, Landos plans to complete remaining IND-enabling studies, file an IND for BT-11 in 2018 and expects to complete Phase 1 clinical testing by early 2019.
For more information about Landos Biopharma, visit www.landosbiopharma.com. For more information about Xontogeny, visit www.xontogeny.com. For more information about Perceptive Advisors, visit www.perceptivelife.com.
About Landos Biopharma, Inc.
Landos Biopharma, Inc. is an emerging biopharmaceutical company focused on the discovery and development of first-in-class oral therapeutics for patients with autoimmune diseases. Landos’ lead clinical asset, BT-11, is a novel, locally-acting small molecule targeting inflammatory bowel disease (IBD) that is expected to enter clinical testing for Crohn’s disease in 2018. Landos also has a robust pipeline of compounds for other autoimmune diseases. Landos is headquartered in Blacksburg, VA. For more information, please visit www.landosbiopharma.com, contact info@landosbiopharma.com or follow the company on Twitter at @LandosBio.
Xontogeny’s Landos Biopharma reels in $60M to ramp up IBD program – Fierce Biotech 8/13/2019
Two years ago, Landos Biopharma was the first biotech to come out of Xontogeny, the accelerator started by former Sarepta Therapeutics chief Chris Garabedian. Now, the autoimmune specialist has picked up $60 million to propel its lead program into phase 2 for inflammatory bowel disease (IBD).
The series B funding will also advance Landos’ earlier-stage pipeline, which includes candidates slated to enter the clinic in 2020. It comes from RTW Investments, Osage University Partners, PBM Capital and Perceptive Advisors—which includes Perceptive Life Sciences Fund and Perceptive Xontogeny Venture Fund.
Landos is based upon the work of CEO Josep Bassaganya-Riera, Ph.D., a serial entrepreneur and Virginia Tech professor who outlined the potential for targeting the lanthionine synthetase c-like protein 2 (LANCL2) pathway to affect immune and inflammatory responses in a 2014 paper. Bassaganya-Riera went on to discover a drug that selectively binds LANCL2, found Landos and pick up $10 million from Perceptive Advisors to drive its lead program.
The program, dubbed BT-11, is a small molecule that targets LANCL2 in the gut. In a phase 1 study published earlier this year, the drug beat placebo at lowering levels of fecal calprotectin, which Landos believes is a predictive biomarker of response to treatment in IBD. The company will kick off global phase 2 trials in the two main forms of IBD: Crohn’s disease and ulcerative colitis.
“We believe BT-11’s mechanism of action is differentiated with the potential to transform the current treatment paradigm for patients with ulcerative colitis and Crohn’s disease,” said Rod Wong, M.D., a managing partner at RTW Investments who has just joined Landos’ board, in a statement.
Landos is looking to provide a new option for patients with moderate to severe cases of IBD for whom current treatments are inadequate or have side effects. People with IBD may be treated with anti-inflammatory drugs or immunosuppressants such as anti-TNF (tumor necrosis factor) meds, but Landos believes the former can be used only in mild inflammation and reckons the latter only works in up to 60% of patients.
“We believe there is tremendous commercial potential for an oral compound for IBD and BT-11 is the most promising candidate we’ve seen at this stage of development,” said Garabedian, portfolio manager at Perceptive Xontogeny Venture Fund. “As an investor in the Series A, we are impressed with the productivity and efficiency of the Landos team in completing a comprehensive preclinical program, securing two open INDs, and successfully generating Phase 1 clinical results in less than two years, and are prepared to move forward with two global Phase 2 studies in ulcerative colitis and Crohn’s disease.”
그리고 이어서 NX-13이라는 두번째 약물을 임상1상에서 첫번째 환자에게 약물복용이 되었다고 발표를 했습니다. 정말 굉장한 추진력입니다.
Landos Biopharma, a clinical-stage biopharmaceutical company focused on the discovery and development of therapeutics for patients with autoimmune disease, today announced that the first patient has been dosed in a Phase 1 study of NX-13, the Company’s novel, orally administered therapeutic candidate for the treatment of inflammatory bowel disease.
“Today marks a significant milestone for Landos, as we advance our second product candidate, NX-13, into clinical development,” commented Josep Bassaganya-Riera, Ph.D., Chairman, President, and CEO of Landos Biopharma. “This achievement highlights the success of our precision medicine platform in identifying novel targets to establish a robust and differentiated therapeutic pipeline for autoimmune disease. NX-13 is a compound for oral administration that targets the NLRX1 receptor, which is part of a pathway that modulates immune response linked to inflammatory bowel disease. We believe that, if approved, NX-13 could provide an additional treatment option to the up to 50 percent of ulcerative colitis patients that experience relapse within 1 year of current therapies and up to 70-90 percent of Crohn’s disease patients that fail to enter prolonged remission.”
The randomized, double-blind, placebo-controlled, ascending dose, multi-cohort Phase 1 study will evaluate the safety, tolerability, and pharmacokinetics of NX-13 in healthy volunteers. The study design includes evaluation of single ascending doses and multiple ascending doses of NX-13. Based on observations in preclinical models of inflammatory disease, NX-13 has the potential to be developed as a monotherapy or in combination with other therapeutics in the treatment of inflammatory bowel disease.
“Advancing two first-in-class oral products for Crohn’s disease and ulcerative colitis with new mechanisms of action into clinical development in less than 2 years is a substantial accomplishment,” said Jean-Frederic Colombel, M.D., a Clinical Advisory Board member for Landos’ IBD program, gastroenterologist and Director of the IBD Center at the Icahn School of Medicine at Mount Sinai. “We continue to see an unmet clinical need for chronic oral therapies to treat UC and CD with improved efficacy, safety, tolerability and convenience, including in the mild to moderate patients.”
About Inflammatory Bowel Disease
Inflammatory bowel disease (IBD) is a chronic autoimmune disorder with two primary subtypes: Crohn’s disease and ulcerative colitis, which impair quality of life and afflict over five million individuals worldwide.1 Currently, available therapeutics typically are only able to address a subset of overall patient populations and many fail to maintain therapeutic efficacy over time. In addition, current therapeutics are associated with serious side effects and toxicities related to systemic immunosuppression, including increased mortality.2
1. World IBD Day. Home. Available at http://www.worldibdday.org/index.html. Accessed July 2020.
2. Shivaji, U.N. et al., Review article: managing the adverse events caused by anti-TNF therapy in inflammatory bowel disease. Aliment Pharmacol Ther. 2019 Mar;49(6):664-680.
About NX-13
NX-13 is a first-in-class, orally-active, gut-restricted, small molecule therapeutic candidate for the treatment of inflammatory bowel disease. NX-13 targets NLRX1, a mitochondria-associated receptor with the ability to modulate immune responses. By activating the NLRX1 pathway, NX-13 increases oxidative phosphorylation in immune cells, reduces differentiation of effector CD4-positive T cells, and decreases production of inflammatory cytokines.
About Landos Biopharma
Landos Biopharma, Inc. is a clinical-stage biopharmaceutical company focused on the discovery and development of first-in-class oral therapeutics for patients with autoimmune disease. Lead asset BT-11 is a novel, first-in-class, oral, gut-restricted therapeutic candidate for the treatment of ulcerative colitis and Crohn’s disease that targets the LANCL2 pathway. NX-13 is a novel, first-in-class, oral, gut-restricted compound for the treatment of inflammatory bowel disease, which targets the NLRX1 pathway. Additional candidates are in development for the treatment of lupus nephritis, rheumatoid arthritis, multiple sclerosis, and diabetes. For more information, please visit www.landosbiopharma.com.
그리고 이듬해에는 $100 Million IPO를 할 수 있었습니다. 여기까지는 모든 것이 다 좋아보였습니다.
Landos Biopharma guns for a $100M IPO to boost AI autoimmune R&D work – Fierce Biotech 1/15/2021
Xontogeny’s Landos Biopharma is jumping into warm biotech IPO waters as it looks to go public with a $100 million offering.
Landos is based upon the work of CEO Josep Bassaganya-Riera, Ph.D., a serial entrepreneur and Virginia Tech professor who outlined the potential for targeting the lanthionine synthetase c-like protein 2 (LANCL2) pathway to affect immune and inflammatory responses in a 2014 paper.
Bassaganya-Riera went on to discover a drug that selectively binds to LANCL2, found Landos and pick up $10 million from Perceptive Advisors to drive its lead program.
Landos Biopharma was the first biotech to come out of Xontogeny, the accelerator started by former Sarepta Therapeutics chief Chris Garabedian. Back in the summer of 2019, it picked up $60 million series B to propel its lead program into phase 2 for inflammatory bowel disease (IBD).
Now, it wants a $100 million IPO, according to its Securities and Exchange Commission filing, to help boost its AI-based LANCE platform, which sees to make predictions of immunometabolic function. To date, it has identified seven novel immunometabolic targets and product candidates across 14 indications.
Its leading candidate, BT-11, has completed the induction phase of a phase 2 trial in mild to moderate ulcerative colitis (UC) patients, with plans to kick-start a phase 3 in UC and a phase 2 for moderate to severe Crohn’s disease in the coming months.
Landos is looking to provide a new option for patients with moderate to severe cases of IBD for whom current treatments are inadequate or have side effects.
People with IBD may be treated with anti-inflammatory drugs or immunosuppressants such as anti-TNF meds, but Landos believes the former can be used only in mild inflammation and reckons the latter only works in up to 60% of patients. It will now seek to out that theory to the test in later-stage trials.
그런데 IPO한 지 얼마되지 않아 Josep 교수는 갑자기 회사를 그만두게 됩니다. 이사진과의 갈등이 원인이 된 것 같죠. 아마 주된 이유는 이후의 일로 보았을 때 BT-11과 NX-13 중에서 어떤 약물을 집중하느냐의 문제였던 것으로 보입니다. Josep 교수가 그만둔 후 Landos는 NX-13의 임상을 진행하고 BT-11은 중단합니다.
Landos Biopharma CEO Josep Bassaganya-Riera leaves – Exchange 11/8/2021
Josep Bassaganya-Riera, chief executive of Landos Biopharma, leaves. As announced by Landos Biopharma Inc. in a news release on Monday, November 8, 2021, Josep Bassaganya-Riera leaves his post as chief executive officer at the clinical-stage biopharmaceutical company, after about five years in the role, effective immediately.
Landos Biopharma will undertake a search for a successor.
Josep Bassaganya-Riera’s duties as CEO will be taken over temporarily by Tim M. Mayleben, most recently President, CEO and Director at Esperion Therapeutics, Inc., as Interim Chief Executive Officer.
Already a director
Mayleben is already a director of Landos Biopharma. Generally speaking, most director-turned-CEO appointments occur following a sudden resignation of the outgoing CEO and signal a lack of preparedness on the company’s part to groom internal talent. Directors-turned-executives represent a blend of outsider and insider.
They don’t have the constraints of a pure insider when it comes to leading painful changes or making unpopular decisions, and they have more company knowledge than a pure outsider.
Having been a director, Mayleben understands the expectations and dynamics of the board and has knowledge of Landos Biopharma’s organization, risk-management practices and strategy.
Chris Garabedian, also a Landos Director, has been appointed Chairman of the Board.
“This is the right time”
Josep Bassaganya-Riera’s departure from the CEO post is explained as follows. Chris Garabedian, Chairman of the Board, said: “With seven product candidates in the pipeline across three novel mechanisms, Josep and the Board agree that this is the right time to transition to our next phase of leadership.”
Precise information regarding Josep Bassaganya-Riera’s future plans was not immediately available.
“Stepped down”
Landos Biopharma said: “Josep Bassaganya-Riera, Ph.D., has stepped down as Chairman, President and Chief Executive Officer, effective immediately.”
Share price increase since June 2021
The announcement follows an increase in Landos Biopharma Inc.’s share price of 30% since June 2021.
In the position of CEO since 2017
Josep Bassaganya-Riera became CEO of the Company in 2017.
Bassaganya-Riera will serve as an advisor to the Company to ensure a smooth transition.
Josep Bassaganya-Riera has served as the Company’s Chairman, President and Chief Executive Officer since the Company’s founding in January 2017.
Bassaganya-Riera has served as the Director of the Nutritional Immunology and Molecular Medicine Laboratory since July 2002 and the Chairman of the board of directors of Biotherapeutics Inc. since October 2008.
He previously served as the Chief Executive Officer of Biotherapeutics from October 2008 to September 2017 and a Research Professor of Immunology at Virginia Tech from December 2002 to May 2020.
Bassaganya-Riera holds a degree in Veterinary Medicine from Universitat Autònoma de Barcelona and a Ph.D. in Nutrition and Immunology from Iowa State University.
하지만 Josep 교수는 여기서 포기하지 않습니다.2023년 3월에 Landos로 부터 NX-13을 제외한 물질을 사게 됩니다. 그리고 NImmune Biopharma를 설립합니다.
Endpoints 의 기사가 좀더 자세히 상황을 설명해 주는 것 같습니다. 약물의 용량을 줄이고 subset disease로 임상3상을 추진한다는 계획을 밝혔습니다. 펀딩에 대해서는 자세한 언급은 없었지만 투자자들이 든든히 받치고 있다고 했다는군요. 펀딩 부분은 조만간 뉴스가 있겠죠.
By the time Josep Bassaganya-Riera stepped down as founding CEO of Landos Biopharma in 2021, the company had racked up Phase II data for its top autoimmune program, completed what he called a positive end-of-Phase-II meeting with the FDA and plans to launch pivotal Phase III trials.
Since then, though, the new leaders at Landos have reshuffled their plans for the drug, omilancor, first announcing they will run a Phase IIb ahead of a Phase III and eventually shelving it altogether.
Now, Bassaganya-Riera is picking back up where he left off.
NImmune Biopharma, his new startup, has bought rights to omilancor and other similar drugs from Landos, with an eye to launching Phase III this year. The company’s name first cropped up in SEC filings when Landos announced the deal in early March, but Bassaganya-Riera is sharing more details about the Phase III design and his team in an official launch.
Joining him in the C-suite are several veterans of Landos, including CSO Raquel Hontecillas, Marek Ciszewski (CFO), Andrew Leber (chief development officer), and Jennifer Collette (chief accounting officer & controller). The executives were formerly Landos’ VP of financial strategy and investor relations, VP of scientific & product development and head of finance, respectively.
The team’s focus remains on a portfolio of drugs targeting LANCL2, a protein that Bassaganya-Riera had researched for years as a professor at Virginia Tech.
While the clinical remission rate shown in the Phase II trial Landos ran in mild-to-moderate ulcerative colitis was not statistically significant, Bassaganya-Riera believes there’s evidence the drug works for a subset of patients with more serious disease.
For the late-stage trial, that means “a refinement of the patient population consistent with the expectations of the FDA.”
“In a nutshell, the expectation of the FDA is that we focus on patients that have more active disease,” he said, adding that NImmune will go for a 440 mg dose, which is lower than the 500 mg and 1,000 mg tested in Phase II.
He declined to offer specifics on fundraising progress so far, saying only there are institutional investors on board that will put NImmune in a well-capitalized position.
NImmune will aim to start other trials for omilancor in Crohn’s disease while testing a second candidate for lupus and rheumatoid arthritis — in line with the broad potential it sees for the LANCL2 pathway in treating autoimmune diseases.
“It’s different from the current Landos, but it’s not that different from the Landos that I founded in 2017,” he said.
NImmune Biopharma (“NImmune”), a late-stage precision immunology biopharmaceutical company that develops best-in-class biomarker-driven immunoregulatory therapeutics, today announced its launch following the acquisition of omilancor, NIM-1324, and the entire LANCL portfolio of immunoregulatory therapeutic assets from Landos Biopharma, Inc.
Omilancor is a Phase-3-ready, once-daily, oral, gut-restricted therapeutic for Ulcerative Colitis (UC) with potentially fast-to-market follow-on opportunities in Crohn’s disease (CD) and Psoriasis. NIM-1324 is a Phase-2-ready biomarker-guided once-daily, oral therapeutic for the treatment of Systemic Lupus Erythematosus (SLE) and Rheumatoid Arthritis (RA). These clinical candidates, originally developed by NImmune’s Founder, Executive Chairman, President, and Chief Executive Officer Dr. Josep Bassaganya-Riera, activate the Lanthionine Synthetase C-Like 2 (LANCL2) pathway, which enhances immunoregulatory processes that provide protection from autoimmune disease.
“I am thrilled to regain ownership and leadership of the LANCL immunoregulatory therapeutic portfolio and look forward to continuing the development of these candidates at NImmune, a science-driven company developing best-in-class biomarker-driven immunoregulatory therapeutics for a growing patient population with autoimmune diseases and unmet medical needs,” stated Dr. Bassaganya-Riera, Founder & CEO of NImmune. “Our leadership team was instrumental in the creation of the LANCL immunoregulatory portfolio, and brings substantial experience working with omilancor specifically to NImmune. Omilancor has the potential to impact the global inflammatory bowel disease (IBD) market as a safe and effective therapy, given the statistically significant clinical remission data it has produced in active disease UC patients as well as promising efficacy and safety results in CD patients. We are highly encouraged and motivated by these results and are pleased to have identified a robust regulatory path to commercialization.”
Omilancor was previously evaluated for the treatment of UC in a Phase 2 randomized, placebo-controlled clinical trial that demonstrated biologic-like efficacy with potentially best-in-class safety. Based on these findings and positive correspondence with the U.S. Food and Drug Administration (FDA), NImmune plans to initiate a Phase 3 randomized, placebo-controlled clinical trial in 2023. This study will be similar in design to the previous study but will only include the 440mg dose and utilize refined criteria for active disease, that includes rectal bleeding (RB) > 0, histological activity and elevated fecal calprotectin (FCP) at baseline. An analysis of the Phase 2 data using the 440mg dose and the refined active disease population attained statistically significant clinical remission at week 12. Meeting the approvable primary endpoint in its planned Phase 3 population of active disease UC patients sets a robust regulatory path for omilancor to New Drug Application (NDA) filing and commercialization.
A summary of the Phase 2 omilancor data can be found in NImmune’s corporate presentation.
NImmune’s second pipeline clinical candidate, NIM-1324, a once-daily, oral, systemically distributed LANCL2 agonist, has demonstrated ability to induce enhanced regulatory T cell (Treg) function in preclinical models of SLE and RA, reduced interferon gamma signaling in human peripheral blood mononuclear cells, and the potential to reduce inflammatory cell infiltration with less toxicity than current standard of care, including biologics and JAK-inhibitors. Additionally, Phase 2-ready NIM-1324 successfully completed a Phase 1 randomized, double-blind, placebo-controlled multi-cohort study evaluating its safety, tolerability, and pharmacokinetics (PK) in normal healthy volunteers where all endpoints were met, and it is now ready for further clinical testing in lupus and RA patients.
About NImmune Biopharma
NImmune is a late-stage precision immunology biopharmaceutical company that develops best-in-class biomarker-driven immunoregulatory therapeutics. Underpinned by advanced computational modeling and bioinformatics coupled with biomedical research capabilities to pioneer innovation in immunoregulatory drug development, NImmune’s business model enables the rapid and capital-efficient clinical development of high conviction drug candidates into New Drug Application (NDA) filing and commercialization. NImmune’s clinical development pipeline includes omilancor, a Phase 3-ready lead clinical candidate targeting LANCL2, an oral, once-daily, gut-restricted, first-in-class therapeutic for Ulcerative Colitis and Crohn’s disease with registration-directed pivotal clinical trials planned for 2023. Additional information: www.NIMMUNEBIO.COM or contact media@nimmunebio.com.
NImmune Biopharma, (“NImmune”), a private late-clinical-stage precision immunology biopharmaceutical company focused on the discovery and development of best-in-class biomarker-driven immunoregulatory therapeutics, led by omilancor, a Phase 3 best in class once daily oral therapy for Ulcerative Colitis, announced that it will present three abstracts, including final Phase 2 data for omilancor in active Ulcerative Colitis patients at the American College of Gastroenterology (“ACG”) 2023 Annual Scientific Meeting (“ACG 2023”), taking place at the Vancouver Convention Center in Vancouver, Canada, between October 20 and October 25, 2023.
Dr. Josep Bassaganya-Riera, Founder & CEO of NImmune, said, “Final and complete Phase 2 data for omilancor in mild to severe UC patients with active disease affirms best-in-class efficacy and unrivaled safety. Omilancor is a first-in-class wholly-owned therapeutic, developed with the guidance of TITAN-X, a proprietary computational modeling and AI-powered precision medicine discovery engine that efficiently accelerates biomarker-driven immunoregulatory therapeutic development of omilancor, NIM-1324 and our other immunoregulatory therapeutics. Meeting the primary endpoint of clinical remission in UC patients with active disease substantially de-risks omilancor’s regulatory path to New Drug Application (NDA) and commercialization and provides clinical validation of the LANCL2 pathway as a novel mechanism of action for addressing the significant unmet clinical needs of patients with autoimmune diseases. These positive clinical findings further underscore the importance of our recently announced research collaboration with the NIMML Institute and the value of its advanced computational modeling and A.I.-powered TITAN-X precision medicine platform, which efficiently accelerates biomarker-driven immunoregulatory therapeutic development of omilancor, NIM-1324 and our other immunoregulatory therapeutics.”
“As we approach a significant milestone—the initiation of the pivotal global Phase 3 clinical program of omilancor for the treatment of UC by year-end 2023—we are pleased to present our results at ACG 2023 and encouraged by the continued scientific validation of our clinical findings and the overall momentum of omilancor’s clinical and regulatory development. We look forward to continuing to realize the significant potential of omilancor as the first-in-class LANCL2 agonist for UC and additional autoimmune indications including Crohn’s disease and psoriasis.”
Presentation Details
Title: Efficacy and Safety of Omilancor in a Phase 2 Randomized, Double-Blind, Placebo-Controlled Trial of Patients with Ulcerative Colitis.
Poster: Board NumberP2216,Monday October 23, 2023, 10:30 AM – 4:15 PM
- Clinical remission in the approvable UC population of active disease mild to severe patients was induced in 30.4% of patients treated with omilancor relative to 3.7% of patients given placebo (Δ=26.7, P = 0.01), meeting the primary endpoint.
- Endoscopic and histological remission were achieved in 41.7% of omilancor given patients relative to 18.6% and 22.2%, respectively, patients in the placebo group (Δ=23.1, Δ=19.5).
- Durable remission was induced in 38.5% of patients in the omilancor group versus 21.4% of patients given placebo (Δ=17.1, P = 0.05).
- Endoscopic response was achieved in 73.1% of patients treated with omilancor relative to 53.6% of patients given placebo (Δ = 19.5, P = 0.02).
- Omilancor exhibited statistically significant decreases in TNF-a expressing myeloid cells (p = 0.037) in the colonic mucosa and statistically significant normalization of fecal calprotectin levels (P = 0.048).
- Oral omilancor was well-tolerated in patients with UC with no trends in AE profile observed and no dose-limiting toxicities.
- Pharmacokinetic analysis validated a gut-restricted profile with stable drug levels in stool over the treatment period, penetration into colonic biopsy tissue and limited systemic exposure.
Title: Efficacy and Safety of Omilancor in a Phase 2 Randomized, Double-Blind, Placebo-Controlled Trial of Patients with Crohn’s Disease.
Poster: Board NumberP2215,Monday October 23, 2023, 10:30 AM – 4:15 PM
- PRO-2 clinical remission was achieved in 41.7% of moderate to severe CD patients in the omilancor group relative to 9.1% give placebo (Δ=32.6).
- Crohn’s disease activity index (CDAI) clinical remission was achieved in 25.0% of omilancor-treated patients relative to 9.1% of the placebo group (Δ=15.9).
- Omilancor demonstrated a promising efficacy signal in both biologic naïve and biologic-experienced moderate to severe CD patients.
- Normalization of fecal calprotectin was induced in 33.3% of patients treated with omilancor compared to 14.3% in the placebo group (Δ=19.0).
- In patients with histological activity in at least one segment at baseline, omilancor induces remission in all segments in 42.9% of patients relative to 20.0% in the placebo group (Δ=22.9).
- Oral omilancor was well tolerated in patients with CD and displayed a benign safety profile.
Title: Transcriptional Analysis of Colonic Biopsies from Patients with Ulcerative Colitis Treated with Omilancor.
Poster: Board NumberP2217, Monday October 23, 2023, 10:30 AM – 4:15 PM
- Predictive biomarker signatures from colonic biopsies were identified by using the RandomForest A.I. algorithm within NIMML’s TITAN-X drug development platform.
- A newly identified precision immunology biomarker signature predicts omilancor responders from non-responders with 75% accuracy and patients treated with omilancor and achieving clinical remission were identified with 100% accuracy.
- Predictive modeling of gene expression changes from baseline accurately differentiated patients treated with omilancor from those given placebo with 83% accuracy.
- Biomarkers upregulated by omilancor were associated with lipid metabolism, ion balance, and known critical elements of the LANCL2 pathway.
- Biomarkers downregulated by omilancor were associated with immune systems processes, mainly linked to neutrophils and leukocyte trafficking.
The posters will be available under the “Publications” section of the NIMML’s website at www.nimml.org and at the ACG 2023 ePoster Hall during and after the meeting. Additionally, the peer-reviewed accepted abstracts will be published verbatim in a special supplement to the October 2023 issue of The American Journal of Gastroenterology.
About Ulcerative Colitis (UC)
UC is a chronic, autoimmune, inflammatory bowel disease that causes inflammation, irritation, and ulcers in the lining of the large intestine (colon) and rectum. Symptoms include abdominal pain, rectal pain and bleeding, bloody stools, diarrhea, fever, weight loss, and malnutrition. Having UC puts a patient at increased risk of developing colon cancer. Diagnosis typically occurs in early adulthood and the disease requires maintenance treatment for the remainder of the patient’s life. UC is estimated to affect over 900,000 patients in the United States and over 1 million patients throughout the rest of the world. With 70% of addressable patients experiencing a second flare within one year and 30% of patients in remission failing to stay in remission for more than one year, there is an unmet medical need in UC for safer and more efficacious therapeutics.
About Crohn’s Disease (CD)
CD is a chronic, autoimmune, inflammatory bowel disease that causes inflammation, irritation and ulcers in any segment of the gastrointestinal tract. CD impacts the end of the small bowel and beginning of the colon most commonly, which in turn can lead to symptoms of abdominal pain, increased abdominal sounds, rectal pain and bleeding, bloody stools, diarrhea, fever, weight loss and malnutrition. There are four classes of CD and treatment depends on the level of severity. Current therapeutic options for severe disease, primarily biologics, have several limitations, which include but are not limited to safety risks for malignancies and infections, limited efficacy and lack of long-term maintenance options. There is an urgent need to establish a consensus for a first-line therapy for CD and improve upon the existing constraints in administration and efficacy.
About Omilancor
By activating the LANCL2 pathway and modulating the interactions between immunological and metabolic signals in immune and epithelial cells, omilancor is a first-in-class, oral, once-daily, gut restricted therapeutic designed to create a favorable regulatory microenvironment in the gut, decreasing the production of key inflammatory mediators and increasing anti-inflammatory functions in regulatory T cells (Treg) within the site of inflammation. Omilancor has completed Phase 2 clinical testing in UC patients showing a clinical remission of 30.4% with a placebo-adjusted 12-week clinical remission rate of 26.7% (p=0.01) for the 440 mg dose. Following demonstration of a statistically significant approvable primary endpoint for clinical remission in an active disease patient population, NImmune expects to initiate a global pivotal Phase 3 program (PACIFY I and PACIFY II trials) in UC patients in the second half of 2023. Omilancor’s target U.S. market size is expected to be valued at $394.9 billion 2021-2030, of which a peak annual market size of $49.5 billion is expected to occur in 2030. NImmune expects peak unadjusted revenue of $12.5 billion in 2030.
About NIM-1324
NIM-1324 is an oral, systemically distributed, small-molecule therapeutic candidate which activates LANCL2, a surface membrane-associated receptor that is responsible for modulating key cellular and molecular changes tied to autoimmune diseases. By activating the LANCL2 pathway, NIM-1324 increases the anti-inflammatory capacity and stability of regulatory CD4+ T cells while also supporting the metabolic demands of autophagy in phagocytes. To date, treatment with NIM-1324 has reduced the production of interferon alpha in human peripheral blood mononuclear cells (PBMCs) from systemic lupus erythematosus (SLE) patients and provided protection from clinical disease and tissue pathology in mouse models of lupus, rheumatoid arthritis, and multiple sclerosis. Phase 2-ready NIM-1324 completed Phase 1 clinical testing where it met all endpoints and demonstrated a dose proportional change in plasma exposure within the therapeutic range with no accumulation. NIM-1324 target U.S. market size is expected to be valued at $226.0 billion 2021-2030, of which a peak annual market size of $23.1 billion is expected to occur in 2030. NImmune expects unadjusted revenue estimates from NIM-1324 therapeutics to be valued at $2.3 billion from the 2028-2030 projections.
About NImmune Biopharma
NImmune is a late-stage precision immunology biopharmaceutical company that develops novel best-in-class biomarker-driven immunoregulatory therapeutics. Underpinned by a discovery platform that utilizes advanced computational modeling, A.I. and bioinformatics coupled with biomedical research capabilities to pioneer innovation in immunoregulatory drug development, NImmune’s business model enables the rapid and capital-efficient clinical development of high conviction drug candidates into New Drug Application (NDA) filing and commercialization. The lead product candidate from NImmune’s internal discovery platform is omilancor, a wholly-owned Phase 3 oral, once-daily, gut-restricted, first-in-class therapeutic targeting LANCL2 for Ulcerative Colitis, with fast follower potential in Crohn’s disease, Psoriasis and other autoimmune diseases. Phase 2 first-in-patient data for omilancor in UC show potential best in class efficacy and safety. To learn more, visit www.NIMMUNEBIO.COM or contact media@nimmunebio.com.
그러는 와중에 Landos Biopharma는 $138 Million에 Abbvie에 팔렸습니다. 신약개발은 개발자의 의지와 추진력이 중요한데 Josep 박사가 이끄는 팀의 추진력이 매우 좋고 의지도 좋습니다. 몇년간의 마음고생이 있었겠지만 다시 일어난 만큼 좋은 결과가 있기를 진심으로 기대해 봅니다.
AbbVie has grabbed a wrench to add on some new autoimmune disease options to its pipeline via the acquisition of Landos Biopharma.
The deal values the oral-therapeutic-focused biotech at $137.5 million, with the terms offering $20.42 per share in cash upon closing. AbbVie is also offering a non-tradeable contingent value right at up to $11.14 per share, or an additional $75 million, subject to certain clinical milestones.
AbbVie’s business development team has been incredibly busy over the past few months, snapping up Cerevel Therapeutics in neuroscience and ImmunoGen in oncology for $8.7 billion and $10.1 billion, respectively. But that left immunology, where AbbVie has a major legacy thanks to Humira that just went off patent.
Executives previewed a plan to look for smaller companies after those major outlays, with President and Chief Operating Officer Robert Michael saying that the team would be on the lookout for some smaller deals with early-stage opportunities. Michael said during a fourth-quarter earnings call in February that “our focus in immunology in terms of BD is really looking for new mechanisms of action that can elevate standard of care, whether monotherapy or in combination. I’d say there’s a lot of interest in combination.”
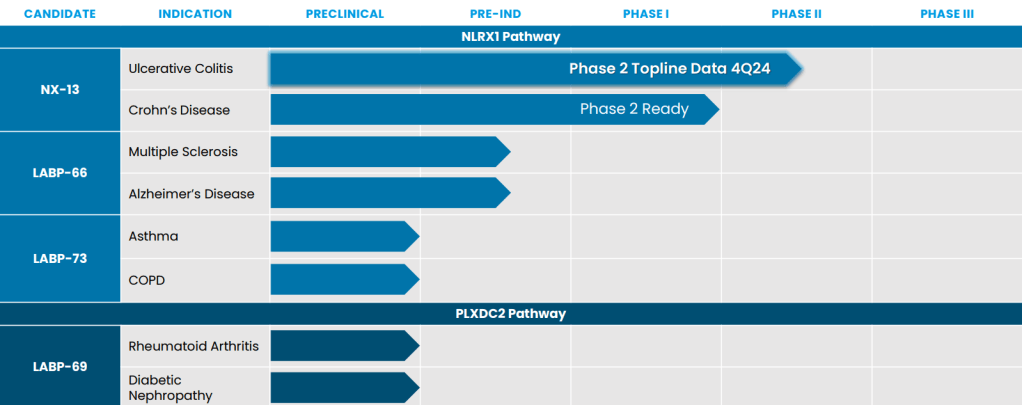
Landos will bring the phase 2 asset NX-13 to the mix, an oral NLRX1 agonist with a bimodal mechanism of action that acts as an anti-inflammatory by facilitating epithelial repair. The biotech is evaluating the treatment in the midstage NEXUS trial in patients with ulcerative colitis (UC). Top-line data are expected in the fourth quarter, according to Landos’ fourth-quarter earnings update issued last week. In addition, NX-13 is being prepped for a phase 2 test in Crohn’s disease.
Also in the pipeline at Landos for the NLRX1 pathway is LABP-66 in multiple sclerosis and other neurodegenerative diseases, plus LAPB-73 in asthma and eosinophilic disorders. The company is studying the PLXDC2 pathway with the early-stage med LABP-69 in rheumatoid arthritis, UC and Crohn’s.
The transaction is expected to close in the second quarter.
2024년 1월에 발표한 Corporate Presentation 자료를 올립니다.