(Picture: John C. Williams, PhD, Professor of City Hope)

(Picture: Ulrich Rodeck, MD, PhD, Professor of Thomas Jefferson University)
Akriveia Therapeutics Raises $7.5M in Series A Funding – FinSME 10/13/2016
Akriveia Therapeutics LLC, a Thousand Oaks, Calif.-based immuno-oncology focused biotechnology company, raised $7.5m in Series A funding.
The round was led by F-Prime Capital Partners. Thomas Beck and Ben Auspitz of F-Prime Capital Partners will join Akriveia’s Board of Directors in conjunction with the funding. Dr. Beck will become Chairman of the Board.
The company intends to use the funds to expand the team to accelerate its therapeutic discovery efforts, and to build a pipeline of single agent and combination immunotherapeutics.
Led by Chief Executive Officer Simon Tomlinson, Akriveia Therapeutics leverages its proprietary platform to create immunotherapeutic products that are specifically activated in the microenvironment of the tumor. To this end, the company also announced that it has entered into exclusive license agreements for key enabling technologies with City of Hope and Thomas Jefferson University.
Akriveia was founded on the work of Professor John Williams at City of Hope and Professor Ulrich Rodeck at Thomas Jefferson University, experts in the fields of bioengineering and cancer therapeutic discovery.
Akriveia Therapeutics Announces Key Additions To Its Leadership Team – Biospace 11/11/2016
Akriveia Therapeutics, an emerging immuno-oncology focused biotechnology company, today announced recent key additions to its research leadership team. Dr. C. Glenn Begley has joined the company as Chief Scientific Officer and Dr. Margaret Karow has joined as Vice President of Discovery and Preclinical Development.
Akriveia Therapeutics is harnessing its proprietary platform to create immunotherapeutic products that are specifically activated in the microenvironment of the tumor. It is anticipated that this will substantially increase the utility of immuno-oncology agents and spare patients the treatment-limiting, debilitating and sometimes lethal side-effects of the current generation of immunotherapeutics.
“Glenn and Margaret joining the company are pivotal events for Akriveia,” commented Dr. Simon Tomlinson, Chief Executive Officer of Akriveia Therapeutics, “the depth and breadth of experience in the discovery and development of cancer biologics that they bring to the company is immense. Their expertise will be key to Akriveia rapidly building a proprietary pipeline of next-generation immunotherapeutics. I am thrilled and honored to welcome them to the company.”
Most recently Dr. Begley was Chief Scientific Officer at TetraLogic Pharmaceuticals. For the 10 years prior he was Vice-President and Global Head of Hematology and Oncology Research at Amgen Inc, based in California. His group was responsible for marketed molecules such as Neupogen, Neulasta, Aranesp, Nplate, Xgeva, discovery of 25 new clinical-stage molecules, and in-licensing of molecules such as Imlygic the first approved oncolytic virus therapy and Blincyto, a bi-specific T-cell engager molecule. Dr. Begley’s personal research has focused on hematopoiesis and stem cell biology. He has published over 200 papers that have been highly cited. His recent work includes analysis of the rigor and reproducibility of scientific research. Dr. Begley originally trained in Australia as a medical oncologist and molecular biologist and has received a number of awards including election to the Association of American Physicians in 2008.
Prior to joining Akriveia, Dr. Karow was an Executive Director at Amgen for 10 years. At Amgen, she held multiple roles in drug discovery and development. These included Executive Director in the Biosimilars Business Unit as the lead for Biosimilars Process Development, and as an Executive Director in Discovery Research leading the Biologics Optimization organization, Protein Sciences at the Thousand Oaks campus, and the site head for the Burnaby, Canada research site where XenoMouse antibody development is based. Prior to Amgen, Dr. Karow was at Regeneron Pharmaceuticals for 10 years, where she was the Vice President of Traps, Small Molecule and Antibody Development, and the Immunology therapeutic area. She was also the co-lead for the development of the VelocImmune mouse platform for the production of human antibodies. As a leader in biotechnology, she has shepherded multiple large molecule projects from their discovery stage and into clinical trials, as well as to the successful filing of marketing applications. Margaret completed her post-doctoral work at Temple Medical School and her Ph.D. at the University of Utah, and holds a BA in MCD Biology from the University of Colorado.
“I’m excited to be joining Akriveia at this strategic stage of its development,” noted Dr. Begley, “the scientific founders have built a powerful and novel platform for the discovery of immuno-therapeutics with pharmacological action targeted to tumor sites. I’m looking forward to helping the company drive the development of a proprietary pipeline and to exploring partnerships that combine our strengths with those of other pharma and biotechnology companies.”
“Akriveia presented a unique opportunity for me to apply my experience of biotherapeutic discovery and development at an early stage company in the immuno-oncology field,” noted Dr. Karow, “the Akriveia approach offers the promise of tackling a significant unmet medical need for safer and more effective immuno-oncology agents. I’m delighted to have joined Akriveia and I am looking forward to helping the company build a pipeline of innovative immunotherapeutics.”
About Akriveia Therapeutics
Akriveia Therapeutics is actively pursuing the discovery and development of the next generation of cancer immunotherapies. Akriveia believes its new agents offer the promise of a safer and more efficacious approach to harness a patient’s own immune system to fight cancer. Akriveia was founded on the pioneering work of Professor John Williams at City of Hope and Professor Ulrich Rodeck at Thomas Jefferson University, experts in the fields of bioengineering and cancer therapeutic discovery. The company is backed by leading life science investor FPrime Capital Partners.
Akriveia Therapeutics, an immuno-oncology focused biotechnology company, today announced its Aklusion™ platform for tumor-targeting cancer immunotherapies will be featured in a poster presentation at the Society for Immunotherapy of Cancer’s (SITC) 32nd Annual Meeting, November 10-12, 2017, at Gaylord National Hotel & Convention Center in National Harbor, MD.
The poster’s authors include scientists from the laboratories of Professor John Williams of the Beckman Research Institute at the City of Hope and Professor Ulrich Rodeck at Thomas Jefferson University, along with Dr. Margaret Karow, Senior Vice President of Drug Discovery and Preclinical Development at Akriveia Therapeutics.
Information about the poster presentation is:
Poster Title: Rational Design of Immuno-Oncology Biologics with Improved Safety and Efficacy
Poster Number: P466
Poster Session: Saturday November 11th 12:30pm-2:00pm and 6:30pm-8:00pm
Akrevia Therapeutics, a privately-held biopharmaceutical company focused on developing highly-potent, tumor-targeted immuno-oncology therapeutics, today announced the closing of a $30 million Series A financing led by F-Prime Capital Partners and Atlas Venture. The investors have come together to recruit an experienced management team to accelerate Akrevia’s pipeline and proprietary Aklusion® platform, which allows therapeutic antibodies, cytokines and chemokines to be specifically activated in the tumor microenvironment and tailored with precisely optimized pharmacologic properties.
Akrevia will be led by Tim Clackson, Ph.D., as President and Head of R&D and Nessan Bermingham, Ph.D., as Executive Chairman. Dr. Clackson joins Akrevia after serving as President of R&D at ARIAD Pharmaceuticals, where he led the discovery and development of Iclusig® (ponatinib) and Alunbrig® (brigatinib), two FDA-approved cancer therapeutics. Dr. Bermingham was most recently CEO of Intellia Therapeutics, a leading gene editing company which he founded, built, and took public.
“We are excited to have the opportunity to work with Tim and Nessan, two experienced drug developers and entrepreneurs who have helped deliver novel therapies to patients and built successful, billion-dollar biotechs,” said Ben Auspitz, F-Prime Capital Partners.
“We were attracted by the strong validation of Akrevia’s founding technology from City of Hope, and the opportunity to harness the therapeutic potential of cytokines and chemokines,” said David Grayzel, M.D., Atlas Venture. “We believe that the Akrevia team and platform will unlock the potential of multiple important immuno-oncology mechanisms and deliver a pipeline of potent, targeted agents to patients.”
Optimizing Potency and Delivery: Aklusion Platform Technology
Akrevia’s Aklusion platform, based on technology licensed from City of Hope and Thomas Jefferson University, allows potently active biological molecules to be inactive until they encounter the tumor microenvironment. With a potential best-in-class anti-CTLA4 antibody as validation, the technology can be applied broadly to other biologic architectures, including highly-potent cytokines and chemokines which currently have limited or no clinical utility due to toxicities. By tailoring pharmacokinetic and pharmacodynamic properties in parallel, Aklusion allows design of molecules with potential best-in-class potency and selectivity.
“Targeting is key to unlocking the true potential of immuno-oncology,” said Tim Clackson, Ph.D., President and EVP R&D, Akrevia Therapeutics. “Currently, we don’t lack potential agents – just the ability to effectively and safely deliver them where they’re needed, and with precisely tailored properties. Our vision at Akrevia is to unleash the full potential of immune stimulating molecules including antibodies, cytokines and chemokines, as new options for patients living with cancer.”
Additional Akrevia leadership includes Ronan O’Hagan, Ph.D., who formerly co-led the oncology discovery program at Merck, as SVP of Discovery, and Margaret Karow, Ph.D., formerly of Amgen and Regeneron, as SVP of Preclinical Development. Thomas Beck, M.D., and Ben Auspitz of F-Prime Capital serve on the Board of Directors alongside David Grayzel, M.D. and Michael Gladstone of Atlas Venture.
About Akrevia Therapeutics
Akrevia Therapeutics, LLC is a privately-held biopharmaceutical company focused on developing highly-potent, targeted immuno-oncology therapeutics. The company’s proprietary Aklusion platform technology allows biologics to be specifically activated in the tumor microenvironment, and with precisely tailored properties, expanding the universe of immune-activating proteins that can be safely delivered. Akrevia is applying its technology to build a broad pipeline of engineered antibodies, cytokines and chemokines as potential new options for patients living with cancer. To learn more, please visit www.akrevia.com.
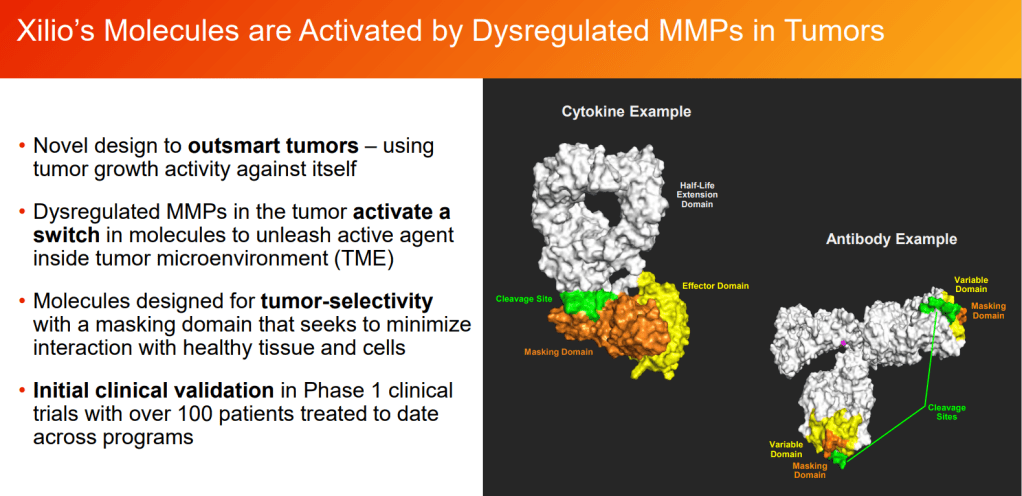
Xilio Therapeutics, a company developing potent, tumor-selective immuno-oncology (IO) therapies for patients with cancer, today announced the closing of a $100.5 million Series B financing. Proceeds from the financing will be used to progress Xilio’s first two therapeutic candidates, XTX201 (tumor-selective IL-2) and XTX101 (tumor-selective aCTLA4 mAb) through Investigational New Drug (IND) enabling studies and into Phase 1 clinical trials, as well as advance additional tumor-selective cytokine programs using Xilio’s proprietary technology.
The financing was led by Takeda Ventures, Inc. with new investors SV Health Investors, MRL Ventures Fund, RiverVest Venture Partners, Bay City Capital, Solasta Ventures, M Ventures, and Ipsen Ventures joining existing investors F-Prime Capital and Atlas Venture in the syndicate.
“We are fortunate to have the support of investors who share our vision to deliver highly potent and effective tumor-selective cancer therapies to patients,” said Rene Russo, Chief Executive Officer of Xilio Therapeutics. “This is a transformational moment for the company as we work to bring our first development programs to patients with cancer and expand our tumor-selective cytokine pipeline.”
Xilio Therapeutics is developing its proprietary technology to create a new class of ultra-potent IO therapies that are activated selectively within the tumor. These tumor-selective therapies are designed to overcome the significant toxicities associated with validated IO therapies, such as IL-2 and aCTLA4, which have historically limited the number of patients that can be treated and prevented patients from completing full courses of treatment. XTX201 (IL-2) and XTX101 (aCTLA4 mAb) have demonstrated tumor-selective activity in preclinical models, significantly widening the potential therapeutic index for these therapies.
“IO has emerged as a major driver of cancer therapeutic development, and agents in this space have proven effective, resulting in compelling durable clinical responses,” stated Jayson Punwani, Investment Partner with Takeda Ventures. “We believe Xilio’s proprietary platform offers a compelling approach that builds upon the advancements in IO therapeutics. It is encouraging to see such a strong and supportive Series B syndicate, including leading venture capital groups and strategic partners. We look forward to working with Xilio’s current investors and the leadership team to support the advancement of its pipeline into the clinic.”
In connection with the closing of the financing, Mr. Punwani, Mike Ross of SV Health Investors, Peter Dudek of MRL Ventures Fund (the therapeutics-focused venture capital group within Merck), and Nancy Hong of RiverVest Venture Partners each joined the Board of Directors of Xilio.
The rebranding of the company from Akrevia Therapeutics to Xilio Therapeutics (pronounced “ex-il-ee-oh”) reflects the company’s evolution from a research-focused organization to a development stage company and commitment to developing the next generation of ultra-potent IO therapies. Xilio is derived from the Latin term Ex Nihilo, meaning creation or big-bang, and embodies the company’s vision to create the next generation of transformative cancer treatments for individuals living with cancer by unleashing the full power of highly potent immune therapies precisely in tumors.
About Xilio Therapeutics
Xilio Therapeutics is a biotechnology company advancing next-generation cancer immunotherapies designed to improve patient outcomes by unleashing the power of the immune system selectively at the site of the tumor. The company’s tumor-selective immunotherapies are based on its proprietary technology, which maximizes the potency of proven immuno-oncology therapies and restricts their activity to the tumor to minimize peripheral side effects. The broad applicability of these therapies across cancer types means that all patients could benefit from these potentially curative medicines.
Xilio was founded in 2016 and is headquartered in Waltham, Mass. For more information, please visit www.xiliotx.com
Xilio raises $95M to take twists on Yervoy and IL-2 into clinic – Fierce Biotech 2/24/2021
Xilio Therapeutics has raised $95 million to take IL-2 and CTLA-4 immunotherapies into clinical trials. The series C positions Xilio to provide early clinical validation of anti-cancer agents that are designed to remain inactive until they reach tumors.
Immuno-oncology drugs are typically given systemically, causing them to trigger immune responses that affect healthy and cancerous tissues alike. The resulting adverse events are unpleasant and can prevent physicians from administering the most efficacious dose. Patients suffer from both the side effects and worse outcomes due to the use of suboptimal doses.
Xilio, formerly known as Akrevia Therapeutics, is built on a platform designed to minimize the effect of cancer drugs on healthy tissues. The potential of the platform has attracted a who’s who of VCs.
Rock Springs Capital led the series C round with support from new backers including Bain Capital Life Sciences, Deerfield Management and RA Capital Management. Existing Xilio investors including Atlas Venture, SV Health Investors and Takeda Ventures also contributed to the financing.
Xilio will use the money to take two drugs, XTX202 and XTX101, into clinical development. XTX202 is a formulation of IL-2, a cytokine that is hamstrung by limitations such as a short half-life and immune effects that necessitate the administration of high doses. XTX101 is a monoclonal antibody against CTLA-4, the immune checkpoint receptor targeted by Bristol Myers Squibb’s Yervoy. INDs for both candidates are planned for this year.
Working with technology licensed from City of Hope and Thomas Jefferson University, Xilio links the drugs to peptides designed to mask the molecules until they reach the tumor microenvironment. The linker is cleaved by proteases found near to tumors, causing the targeted unmasking of the drug.
The approach holds particular promise for potentially highly efficacious molecules that must be given at reduced doses due for safety and tolerability reasons. Xilio thinks IL-2 and CTLA-4 fit the bill.
“While high dose rhIL-2 has the potential for durable complete responses, it is only given to a small number of patients due to its life-threatening toxicity. Similarly, approved aCTLA4 therapy produces severe autoimmune toxicity, limiting potential benefit by preventing patients from receiving highly therapeutic doses or completing full courses of treatment,” Martin Huber, M.D., Xilio chief medical officer, said in a statement.
Xilio Therapeutics Announces Closing of Initial Public Offering – Globe News Wire 10/26/2021
Xilio Therapeutics, Inc. (Xilio) a biotechnology company developing tumor-selective immuno-oncology therapies for patients with cancer, today announced the closing of its previously announced initial public offering of 7,353,000 shares of its common stock at a price to the public of $16.00 per share. The aggregate gross proceeds to Xilio from the offering were approximately $117.6 million, before deducting underwriting discounts and commissions and estimated offering expenses payable by Xilio. In addition, Xilio has granted the underwriters a 30-day option to purchase up to an additional 1,102,950 shares of its common stock at the initial public offering price less underwriting discounts and commissions.
The shares began trading on the Nasdaq Global Select Market on October 22, 2021 under the ticker symbol “XLO.”
Morgan Stanley, Cowen and Guggenheim Securities acted as joint book-running managers for the offering. Raymond James acted as lead manager for the offering.
Gilead Sciences is bucking the trend in IL-12, handing Xilio Therapeutics $43.5 million upfront to get into the space after seeing rivals such as AstraZeneca and Bristol Myers Squibb retreat from the cytokine. Xilio shared the news alongside details of plans to pull back from another program and lay off 21% of its staff.
The Xilio deal gives Gilead a global license to develop and commercialize XTX301, Xilio’s tumor-activated IL-12. Like its sibling IL-2, the cytokine is a potentially potent immunotherapy, but efforts to realize that promise have repeatedly run into setbacks. Drug developers have come up with a range of ways to try to address the toxicity and half-life problems but are yet to deliver an approved IL-12 product.
Xilio’s attempt to crack the challenge rests on the addition of domains for masking and half-life extension to IL-12. Coupled to the domains, IL-12 could travel harmlessly around the body until it enters the tumor microenvironment, where an enzyme removes both add-ons and thereby activates the cytokine.
The biotech is putting that idea to the test in a phase 1 clinical trial. Patients with advanced solid tumors are receiving XTX301 every three weeks. In January, the biotech told investors the drug was generally well tolerated into the third dose level, with no dose-limiting toxicities, and committed to providing safety, pharmacokinetic and pharmacodynamic data in the second half of 2024.
Gilead has made its move ahead of the data drop, handing Xilio $30 million in cash and investing $13.5 million in the biotech in return for an exclusive global license. Xilio is responsible for running the phase 1 trial through dose escalation. Once Gilead receives the data package, it can choose to pay $75 million to take responsibility for further development.
By the time Gilead makes that decision, Xilio could have received up to $29 million more from the Big Biotech in the form of additional equity investments and a development milestone payment. The fees are part of a package of milestones and investments worth up to $604 million. Shares in Xilio, which have languished below $1 this year, shot up by more than 200% in the wake of the update to exceed $2.
The agreement moves Gilead into a space that some of its peers have entered and exited in recent years. Across two updates in 2022 and 2023, AstraZeneca dropped a Moderna-partnered mRNA prospect that encodes for IL-12 and axed an oncolytic viral agent engineered to include a transgene-encoding IL-12. In between those events, BMS returned the rights to Dragonfly Therapeutics’ IL-12 therapy.
Dragonfly is continuing to study its IL-12 candidate in a phase 1/2 trial, making it part of a pack of drug developers that have kept the faith in the cytokine. The pack includes Cullinan Oncology, Philogen, PDS Biotech, Sonnet BioTherapeutics and Xencor.
Gilead is poised to take the baton from Xilio and carry XTX301 through the rest of the race against those rivals. The transfer will leave Xilio focused on its CTLA-4 prospect XTX101, phase 2 data on which are due later this year. Xilio has a third clinical-phase asset, the IL-2 candidate XTX202, but it has decided to stop investing in monotherapy development.
Xilio disclosed the decision alongside new phase 2 data on XTX202 in patients with kidney cancer and melanoma. Stable disease continued to be the best response, leading the biotech to “explore strategic opportunities” for further development in combinations. With its focus narrowing, Xilio is parting ways with 15 employees, representing a 21% reduction of its workforce.