
(Picture: Rudolf Jaenisch & Richard A. Young, Whitehead Institute)
안녕하세요 보스턴 임박사입니다.
Omega Therapeutics는 2015년에 Whitehead Institute의 Rudolf Jaenisch 교수와 Richard A. Young 교수에 의해 Cell Stem Cell에 발표한 Insulated Genomic Domains (IGDs)를 이용해서 Epigenomic Programming을 할 수 있다는 아이디어로 2017년에 Flagship Pioneering의 David Barry 박사에 의해 설립되었습니다.

CAMBRIDGE, Mass. (December 10, 2015) –Whitehead Institute researchers have created a map of the DNA loops that comprise the three dimensional (3D) structure of the human genome and regulate gene expression in human embryonic stem (ES) cells and adult cells. The location of genes and regulatory elements within this chromosomal framework could help scientists better navigate their genomic research, establishing relationships between mutations and disease development.
“This is transformational,” says Whitehead Member Richard Young. “This map allows us to predict how genes are regulated in normal cells, and how they are misregulated in disease, with far greater accuracy than before.”
In order to regulate gene expression, a regulatory element needs to contact its target gene. Through looping, element/gene partners that are distant from each other in linear DNA can be brought together. Most disease mutations occur in regulatory elements, but if the partnership between a seemingly far-flung gene and the regulatory element is not known, the mutation data is of limited use. This draft map, which can help scientists predict the relationships between mutated elements and their target genes, is described online this week in the journal Cell Stem Cell.
“When thinking about disease, we need to think about the structure of the genome in 3D space because that is how we now understand that genes are regulated,” says Xiong Ji, a postdoctoral researcher in the Young lab and a co-author of the Cell Stem Cell paper.
One of Ji’s co-authors, graduate student Daniel Dadon, agrees. “This three-dimensional information helps us to interpret regulatory and mutational data with unprecedented accuracy. It’s not just a bag of genes and regulatory elements in the nucleus—this is a highly organized structure that confers function.”
Previous research in mouse ES cells by Young’s lab and others determined that a chromosome’s DNA is formed into loops that are anchored at their bases by proteins called CTCFs. The benefits of the loops are two-fold. First, the loops help organize and package two meters of DNA to fit into a nucleus that is approximately 5 micrometers in diameter. Second, each loop creates an insulated neighborhood that restricts the action of a regulatory element to genes that resides in the same loop. As graduate student and co-author Diego Borges-Rivera states, “The genome’s 3D shape is a key mechanism underlying gene regulation.”
By studying human ES cells, scientists in the Young lab and the lab of Whitehead Founding Member Rudolf Jaenisch created an initial genome map consisting of 13,000 loops established by CTCF anchors and determined that the average insulated neighborhood is 200 kb in length and contains a single gene. The team found that most of the the mapped CTCF anchor sites in the human ES cells genome are maintained in other human cell types and furthermore, that these loop anchor sequences are highly conserved in primate genomes. Such a surprising degree of conservation indicates that these neighborhoods create a foundational framework for gene regulation that is maintained throughout development and across species.
In a further finding that underscores the importance of the genome’s 3D structure in human health, the Whitehead team found that the CTCF anchor regions are mutated in a broad spectrum of cancer cells. The team predicts that these new maps of the human genome will provide the foundation for improved understanding of the genetic alterations that cause many additional diseases.
This work was supported by the National Institutes of Health (NIH grants HG002668 and HD 045022), National Cancer Institute (NCI), the Erwin Schroedinger Fellowship (J3490) from the Austrian Science Fund, and the Simons Foundation (SFLIFE 286977). Jaenisch is a founder of Fate Therapeutics and Young is a founder of Syros Pharmaceuticals.
2017년 7월부터 2019년 6월까지 우선주 투자방식으로 $28 Million Series A를 받았습니다.
Omega Therapeutics Series A Preferred Stock Financing – Omega Therapeutics S-1 Form 8/1/2017
Series A Preferred Stock Financing. From August 2017 to June 2019, we issued and sold to investors in private placements an aggregate of 56,775,232 shares of our Series A preferred stock at a purchase price of $0.50 per share, for aggregate consideration of approximately $28.4 million.
그리고 2019년 9월에 처음으로 회사의 존재를 세상에 알렸습니다. 2년여의 Stealth mode를 거친 후 발표를 한 것이죠. 이 당시에는 모든 프로그램이 전임상 단계였습니다.
Flagship unveils ‘genome-tuning’ biotech Omega Therapeutics – Fierce Biotech 9/23/2019
Flagship Pioneering launched Omega Therapeutics, a company aiming to take genomic medicine “to the next level.” Founded on the work of two MIT professors, the company is working on treatments that adjust gene expression up or down without making permanent changes to the genome.
Richard Young, Ph.D., and Rudolf Jaenisch, M.D., first described how 3D closed loops of DNA control genomic activity in 2015. Long strands of DNA make these loops because they have to fit into the cell’s nucleus—the loops help “organize and package two meters of DNA” to fit into a space that is about 5 micrometers, or five millionths of a meter, across, the researchers said in a statement at the time. Each loop is an “insulated neighborhood” of one or more genes and their regulatory elements.
Omega is targeting these neighborhoods, called Insulated Genomic Domains (IGDs), with a platform that could be applied to a variety of ailments.
“If you think about it, other than some viral and other infections, pretty much all human disease is due to the dysregulation of genomic expression,” Omega CEO Mahesh Karande, a Novartis alum and former CEO of Macrolide, told FierceBiotech. “Disease mostly occurs because of dysregulation of the genome, by genes not being expressed at the right level. They’re over- or under-expressed. We are able to tune that expression to the native level it’s supposed to be at.”
The Cambridge, Massachusetts-based biotech is mapping IGDs to different diseases and figuring out which of these neighborhoods plays a role in different diseases. From there, it will create treatments it calls Omega Controllers that adjust gene expression to healthy levels.
This adjustment happens without making permanent changes to the genome by switching genes on or off, cutting disease-causing genes out or putting in a healthy version of a faulty gene.
“In nature, generally things are not all the way on or all the way off, but rather turned to a very specific range in a healthy setting,” said Omega Chief Scientific Officer Thomas McCauley, Ph.D., the former CSO of Macrolide and Translate Bio. “Our Omega Controllers are able to target IGDs using the map that Mahesh mentioned and target the right place on that IGD to restore gene function at the right level.”
Because the approach could work for so many disease areas, Omega plans to ink some partnerships as well as work on its own pipeline, said David Berry, M.D., Ph.D., a general partner at Flagship, in a statement. Omega’s treatments could be used to boost the efficacy of in vivo and ex vivo therapies, he said.
In the in vivo space, checkpoint inhibitors are a potential candidate.
“Many of them are not as effective as you’d like them to be. Sometimes immuno-oncology agents act only on 30% of the patient population,” Karande said.
Omega could identify specific genes in patients that affect how they respond to these treatments. For example, if a gene is expressed in patients who don’t respond to a certain immuno-oncology drug, Omega might knock down the expression of that gene to make that drug more effective. On the ex vivo side, Karande envisions Omega’s technology being used when cell therapies are being engineered outside the body.
이듬해인 2020년에 $85 Million Series B를 하면서 임상진입을 시도한다고 발표를 했습니다.
Less than a year after launch, Omega Therapeutics is getting an $85 million cash boost. It will push a pipeline of treatments toward the clinic as well as bankroll the identification of new targets for genomic medicines.
“We had founded Omega with a long-term vision to create a controllable epigenomic programming platform,” Omega CEO Mahesh Karande told Fierce Biotech. Rather than switching genes on and off, cutting out disease-causing genes or replacing them with healthy versions, Omega’s platform is designed to adjust gene expression to healthy levels.
The company’s work is based on “neighborhoods” of genes and their regulatory elements found in loops of DNA called Insulated Genomic Domains (IGDs). These loops occur because long strands of DNA need to fit into the cell’s nucleus.
“In nature, generally things are not all the way on or all the way off, but rather turned to a very specific range in a healthy setting,” Omega Chief Scientific Officer Thomas McCauley, Ph.D., said in a previous interview. Omega’s “epigenomic controllers” are designed to target the right place on specific IGDs to restore gene function at the right level, he said.
Since launch, Omega has been working to figure out which neighborhoods play a role in different diseases.
“We could have gone in various directions,” Karande said. But Omega landed on a handful of areas. It’s advancing five programs spanning oncology and inflammation as well as autoimmune, metabolic and rare genetic diseases, the first of which should hit the clinic in 2021.
In addition to tweaking gene expression without making permanent changes to the genome, Omega’s approach offers advantages over a small-molecule approach to epigenetics.
“There are a number of companies developing small-molecule therapies for epigenetic targets, almost exclusively in cancer,” McCauley.
“The issue is really specificity, in having those molecules go everywhere in the body as opposed to having them go to specific cell types and specific locations in the genome,” McCauley continued, adding that the benefits of such treatments might outweigh the risks in oncology but that this risk-benefit profile may be unacceptable in other diseases.
In its first efforts, Omega is going after targets with links to specific diseases that are well understood, McCauley said. Moving forward, it will take advantage of the lessons it learns to look for new targets.
“We’re looking for the ability to expand laterally,” he added.
One of those lateral expansions could be into COVID-19. Since inflammation plays a big role in COVID-19 infection, Omega could leverage the work it’s already done in that space to quickly move into drug development against the new coronavirus.
Right now, it’s all systems go with its five—potentially six—programs. Karande said the company would be “remiss” if it did not ink partnerships.
“We are absolutely open to partnering with people. We have a robust discovery platform that has many, many more targets in the pipeline, so yes, partnering is definitely in the cards for us,” he said.
그리고 2021년에는 $126 Million 펀딩을 통해서 OTX-2002 (HCC) 약물의 임상진입과 다른 전임상 약물의 개발을 발표했습니다.
Omega Therapeutics is taking it up a notch. The “genome-tuning” biotech raised $126 million to get its lead program, a treatment for liver cancer, into the clinic as well as to advance a clutch of other preclinical prospects including a treatment for acute respiratory distress syndrome (ARDS), a life-threatening lung injury that can result from COVID-19 infection.
The company is working on a new class of treatments called “epigenomic controllers,” which are designed to adjust the expression of target genes. Unlike gene therapies and gene editing approaches that switch genes on and off, cut out disease-causing genes or replace faulty genes with their healthy versions, Omega’s treatments tune gene expression up or down without making permanent changes to DNA.
Its work is based on “neighborhoods” of genes and their regulatory elements found in loops of DNA called Insulated Genomic Domains (IGDs). These loops occur because long strands of DNA need to fit into the cell’s nucleus. Omega’s “epigenomic controllers” are designed to restore gene function to healthy levels by targeting the right place on specific IGDs.
The series C financing follows an $85 million B round in July 2020, which went toward identifying new targets and driving several programs across multiple disease areas through preclinical development.
“When we started, we needed to explore the depth of the platform—we didn’t want to pigeonhole ourselves,” said CEO Mahesh Karande. “That’s how we delineated eight programs at five different targets.”
Omega has started IND-enabling studies for its lead program, OTX-2002, an epigenetic controller programmed to control expression of c-myc, an elusive cancer-driving gene. It is developing the treatment for hepatocellular carcinoma, the most common form of liver cancer.
Unlike Gilead’s antiviral Veklury (remdesivir) and anti-SARS-CoV-2 antibodies from Eli Lilly and Regeneron, Omega’s COVID-19 treatment focuses on ARDS, which is caused by an inflammatory response in the lungs.
“We treat diseases created by functional or structural changes in IGDs and ARDS creates a functional change in a multigenic IGD where cytokines get supercharged and expressed,” Karande said. With its epigenomic controller, Omega aims to reduce the expression of those cytokines.
The company hopes the treatment will fill a gap in COVID-19 treatment.
“The standard of care [for ARDS] is quite insubstantial and largely palliative, involving mechanical ventilation and where possible, steroids,” said Omega Chief Scientific Officer Thomas McCauley, Ph.D.
Besides liver cancer and ARDS linked to COVID-19, Omega is focusing on regenerative medicine, inflammatory diseases, alopecia, non-small cell lung cancer and a group of skin conditions called neutrophilic dermatoses.
The financing will also bankroll a manufacturing scale-up as well as the expansion of Omega’s technology. As it develops drug candidates, it will continue to improve its platform, “taking the guesswork out of it” and making sure it can keep generating new drugs in a reliable and replicable way, Karande said.
As it continues its quest toward the clinic, Omega will build up its workforce, particularly its clinical organization and manufacturing unit.
그리고 같은해 7월에 $126 Million IPO를 했습니다.
Omega Therapeutics Announces Pricing of Initial Public Offering – PR Newswire 7/29/2021
Omega Therapeutics, Inc. (Nasdaq: OMGA) (“Omega”), a development-stage biotechnology company leveraging its OMEGA Epigenomic Programming™ platform to harness the power of epigenetics to develop a new class of DNA-sequence-targeting, mRNA-encoded programmable epigenetic medicines, today announced the pricing of its initial public offering of 7,400,000 shares of its common stock at a price to the public of $17.00 per share. All of the shares of common stock are being offered by Omega. The gross proceeds from the offering, before deducting underwriting discounts and commissions and estimated offering expenses payable by Omega, are expected to be approximately $125.8 million, excluding any exercise of the underwriters’ option to purchase additional shares. Omega’s common stock is expected to begin trading on the Nasdaq Global Select Market under the ticker symbol “OMGA” on July 30, 2021. The offering is expected to close on August 3, 2021, subject to satisfaction of customary closing conditions. In addition, Omega has granted the underwriters a 30-day option to purchase up to an additional 1,110,000 shares of common stock at the initial public offering price less underwriting discounts and commissions.
Goldman Sachs & Co. LLC, Jefferies LLC and Piper Sandler are acting as joint book-running managers of the offering. Wedbush PacGrow is acting as lead manager.
2023년에 OTX-2002의 8명의 환자에 대한 Preliminary clinical trials 결과를 발표했는데 Genetic selectivity와 MYC 가 dose-range에 맞게 감소하는 것을 보고함으로써 플랫폼의 기술이 임상에서도 작용을 한다는 것을 일단 소규모 임상에서 증명을 했습니다.
The investigational mRNA therapeutic OTX-2002 demonstrated encouraging safety, tolerability, and pharmacokinetics in a small population of patients with hepatocellular carcinoma (HCC) and other solid tumors related to the c-MYC (MYC) oncogene, according to a press release on findings from the phase 1/2 MYCHELANGELO I trial (NCT05497453).1

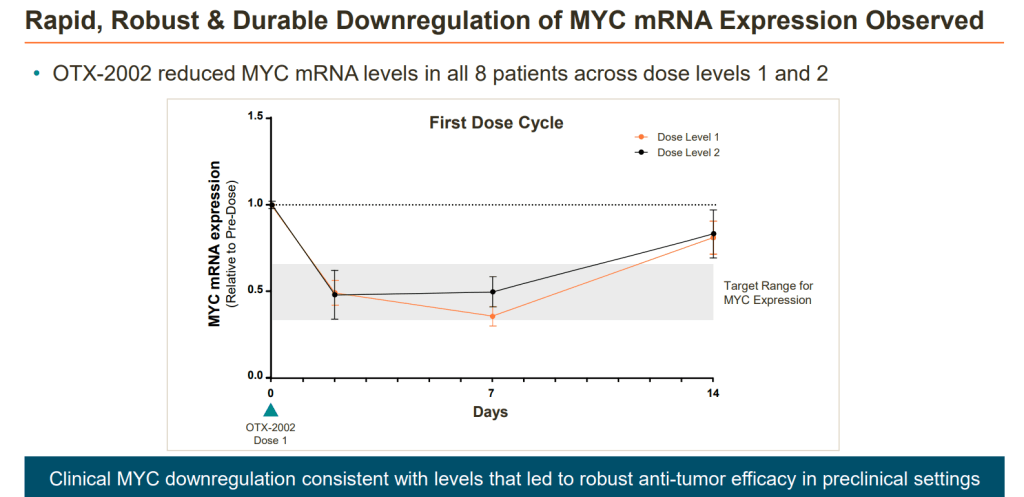
Investigators reported highly specific on-target engagement and epigenetic changes among all 8 patients receiving 0.02 mg/kg (n = 4) or 0.05 mg/kg of OTX-2002 (n = 4) every 2 weeks. The agent’s modulation of MYC rapidly and durably downregulated MYC oncogene expression in all 8 patients; investigators highlighted a mean reduction of 55% at 7 days following treatment.
Treatment with OTX-2002 also produced consistent pharmacokinetic data at both dosing levels, as investigators observed little variability and quick clearance within patients. Additionally, there was no accumulation after additional doses of OTX-2002, and the agent produced low and transient levels of immune response with no adverse effects (AEs) impacting pharmacokinetics. Investigators reported that both initial dose levels of the agent demonstrated anti-tumor activity below the predicted threshold established by preclinical models.
OTX-2002 was well tolerated among patients, and investigators observed no dose-limiting toxicities. Patients mostly experienced grade 1 or 2 AEs, the most common of which included infusion-related reactions (26%) such as fever and chills. These toxicities appeared to be comparable with the known profiles of other FDA-approved agents administered via lipid nanoparticles.
“We are thrilled to see that all 8 patients evaluated at these initial low doses demonstrated clear evidence of on-target epigenetic changes and correlated rapid, robust and durable decreases in MYC mRNA expression levels,” Thomas McCauley, PhD, chief scientific officer at Omega Therapeutics, said in the press release. “These early clinical data are consistent with our preclinical experiments, giving us confidence that our approach has the potential to translate to anti-tumor activity and clinical benefit. Coupled with encouraging safety and predictable pharmacokinetics, we believe that OTX-2002 holds transformative potential for patients living with HCC.”
In the ongoing open-label MYCHELANGELO I trial, investigators are assessing the safety, tolerability, pharmacokinetics, and preliminary anti-tumor activity of OTX-2002 on its own in part 1 and with standard-of-care treatments in part 2 among those with relapsed/refractory HCC and other solid tumor types associated with MYC oncogene expression. Investigators are conducting the trial at clinical sites in the United States and Asia. As of the data cutoff point of September 18, 2023, a single patient with HCC remained on treatment in the 0.05 mg/kg cohort.
The trial’s primary end points are dose-limiting toxicities, treatment-emergent AEs, overall response rate, and duration of response.
Patients 18 years and older with metastatic, advanced, or recurrent solid tumors that have progressed following standard-of-care therapy and intermediate or advanced stage, Child-Pugh A HCC not amenable to locoregional therapy or curative treatment approaches are able to enroll on the study. Having an ECOG performance status of 0 or 1 is another requirement for enrollment.
The FDA granted orphan drug designation to OTX-2002 for managing HCC in November 2022.2
“We look forward to continuing to work with clinical investigators, patients, and the FDA as we advance our MYCHELANGELO clinical program and evaluate the potential of OTX-2002 to bring a new treatment option to the community [of patients with liver cancer],” Mahesh Karande, president and chief executive officer at Omega Therapeutics, said in a press release at the time of the orphan drug designation.2
References
- Omega Therapeutics announces promising preliminary clinical data for OTX-2002 from ongoing MYCHELANGELO™ I trial. News release. Omega Therapeutics. September 26, 2023. Accessed September 26, 2023. https://shorturl.at/hmqNZ
- Omega Therapeutics receives orphan drug designation for OTX-2002 for the treatment of hepatocellular carcinoma. News release. Omega Therapeutics. November 2, 2022. Accessed September 26, 2023. http://bit.ly/3fqiafd
금년 새해 첫날에 Flagship Pioneering과 Novo Nordisk의 계약에 의해서 Omega Therapeutics의 IGD Platform을 이용해서 Obesity 치료제를 개발하는 공동계약을 맺었고 계약 규모는 $532 Million입니다.
As part of a pact with Flagship Pioneering, Novo Nordisk has inked separate cardiometabolic disease research deals with two Flagship-founded biotechs that are worth up to $532 million each.
The freshly formed agreements are with Omega Therapeutics and Cellarity, two Massachusetts biotechs that fall under Flagship’s umbrella. The partnerships are part of a broader ecosystem collaboration Flagship and Novo Nordisk’s Bio Innovation Hub struck up in mid-2022 that aims to quickly build a portfolio of breakthrough medicines for cardiometabolic and rare diseases.
Using Novo’s disease expertise and technology from Flagship’s bioplatform companies, the goal is to generate three to five research programs within the first three years of the partnership, Novo Nordisk’s head of the Bio Innovation Hub Uli Stilz, Ph.D., told Fierce Biotech in an interview.
For these first two deals, each company, Novo Nordisk and Flagship’s Pioneering Medicines—an initiative that develops treatments by using and expanding Flagship innovations—will work together to advance their respective programs through preclinical development. Novo will then have the chance to take the programs into the clinic.
The Big Pharma will reimburse R&D costs and give each company and Pioneering Medicines the chance to make up to $532 million dollars in upfront and milestone payments, plus tiered royalties.
With Omega, Novo will look to expand upon its blockbuster obesity franchise (does the drug Ozempic ring a bell?) with the biotech’s platform, which is made to design programmable epigenomic mRNA medicines that precisely target and modulate gene expression at the pre-transcriptional level.
“We have been pioneers over a 20-year journey in obesity and we want to continue to be a pioneer,” Stilz said, adding that being a pioneer means entering uncharted scientific territory, which is where Omega comes in.
The biotech will use its platform technology to develop an epigenomic controller as part of a new obesity management approach. While many existing therapeutics for weight management focus on appetite regulation, Omega wants to target thermogenesis, a natural metabolic function that regulates overall energy balance.
“What is so exciting for us is that it’s a very different approach than what we have been doing so far,” Stilz said. “I haven’t seen something similar anywhere else. So, we’re really pushing the boundary of science and innovation through this co-creation and collaboration.”
Omega already has some proof-of-concept to support its mission to design programmable mRNA medicines by replicating how nature’s control system works, Omega President and CEO Mahesh Karande told Fierce Biotech. The company’s platform is applicable across almost every disease process, Karande said, and Omega is currently evaluating one of its assets in a phase 1/2 trial for patients with hepatocellular carcinoma.
Now, the company will put its platform to work to control metabolic activity and potentially develop a more durable approach to obesity management.
“Epigenomic control and epigenomic controllers have not existed before. We have literally created this field,” Karande said. “And now we have signed our first major agreement with a company that is an expert in obesity, metabolics and cardiovascular. So, for us, this is hugely validating.”
Meanwhile, Cellarity will plug away at creating a small molecule therapy to treat metabolic dysfunction-associated steatohepatitis (MASH)—the new term for nonalcoholic steatohepatitis (NASH)—a chronic and progressive liver disease for which there is no currently approved treatment. The indication has a high unmet patient need, with only four investigational treatments ever making it into phase 3 development for MASH, led by Madrigal Pharmaceutical’s resmetirom, which is awaiting an FDA decision this spring.
Cellarity and Novo hope to develop a small molecule therapy for the indication by using the biotech’s platform that is designed to provide new insight into cellular dysfunction and allow for drug creation that has been historically inaccessible using traditional drug discovery methods.
Cellarity combines biology, chemistry and AI machine learning to understand cell behavior, Novo’s Stilz explained. Novo Nordisk has previously connected with Cellarity, asking the biotech in September 2022 to identify novel cell behaviors involved in MASH disease progression, work that will now be expanded upon under the new research collaboration.
그리고 얼마전에 Omega Therapeutics는 현재 임상 중인 OTX-2002와 전임상 중인 몇개의 약물에 집중한다는 전략적 우선순위 결정을 하면서 35%의 인원 감축을 하는 구조조정안을 발표한 상황입니다. 일단 플랫폼의 초기 임상이 성공하는 것이 중요하기 때문에 올바른 결정을 한 것으로 보입니다. 좋은 결과가 있기를 바랍니다.

Omega Therapeutics, Inc. (Nasdaq: OMGA) (“Omega”), a clinical-stage biotechnology company pioneering the development of a new class of programmable epigenomic mRNA medicines, today announced financial results for the fourth quarter and full year ended December 31, 2023, and a strategic prioritization initiative to focus resources on near-term milestones to support long-term shareholder value.
“2023 was an important year for Omega where we executed to plan and demonstrated clinical validation of an epigenomic controller to regulate c-MYC in humans for the first time. These proof-of-platform clinical data, coupled with our research collaboration with Novo Nordisk in obesity, support the ability of the OMEGA platform to potentially address epigenomic regulation of almost all human genes across broad therapeutic areas including cancer, cardiometabolic conditions and liver regeneration,” said Mahesh Karande, President and Chief Executive Officer of Omega Therapeutics. “Initial clinical data from our ongoing Phase 1/2 MYCHELANGELO I trial of OTX-2002 demonstrated controlled modulation of MYC expression levels, one of the most challenging gene targets in oncology, and an encouraging disease control rate and stable disease in heavily pre-treated, late-stage HCC patients. We are within what we believe is a clinically meaningful dose range and, as we continue to see a promising safety profile for OTX-2002, have recently opened enrollment of Cohort 5. We look forward to sharing additional updates from this program throughout 2024.”
“Today we also announced a strategic prioritization, implemented to ensure we have sufficient resources to advance our lead program and maximize near- and long-term value creation from our platform. As part of this initiative, we are taking difficult but necessary actions to streamline our team and optimize our R&D efforts and cost structure to extend our cash runway into the first quarter of 2025. These changes will unfortunately affect a number of our colleagues, and we are grateful for their dedication and contributions to our mission,” continued Mr. Karande. “As we sharpen our focus, we look forward to the opportunities ahead to generate meaningful clinical data for OTX-2002, continue to demonstrate the broad potential of our platform, and establish additional partnerships. We remain steadfast in our mission to pioneer a new class of programmable epigenomic mRNA medicines to transform the treatment of a broad range of diseases.”
Recent Highlights and Key Anticipated Milestones
Development Pipeline and Platform
- Advanced the Phase 1/2 MYCHELANGELO™ I clinical trial evaluating OTX-2002 in patients with hepatocellular carcinoma (HCC):
- OTX-2002 continues to advance in monotherapy dose escalation.
- As of March 24, 2024, data from the first three cohorts (0.02 mg/kg – 0.06 mg/kg) showed:
- OTX-2002 continued to be generally well tolerated, with no dose-limiting toxicities observed.
- Consistent dose-dependent pharmacokinetics with no drug accumulation observed following repeat doses.
- All patients demonstrated controlled modulation and downregulation of MYC mRNA expression, an important oncogene regulating cell function and cell death.
- The interim disease control rate (DCR) for the target population of HCC patients was 80%, reflecting 4 out of 5 efficacy-evaluable patients having a best overall response of stable disease. These patients had an average of three or more previous therapies and entered the trial with a life expectancy of less than 12 weeks. The DCR for patients with non-HCC solid tumors in the trial (n=5) was 40%, indicating the potential specificity of OTX-2002 for HCC.
- The Company continues to evaluate patients with HCC in Cohort 4 at the 0.12 mg/kg dose level, which recently cleared the 28-day dose limiting toxicity (DLT) window. Based on preclinical experience and modeling, Omega believes this dose level is within the expected active dose range. In March 2024, the Company opened enrollment for Cohort 5 at a dose level of 0.3 mg/kg.
- Omega expects to report additional updated clinical data from monotherapy dose escalation in mid-2024.
- The Company plans for expansion into monotherapy and combination settings in mid-2024.
- Announced research collaboration with Novo Nordisk to develop a novel therapeutic for obesity management:
- The collaboration will leverage Novo Nordisk’s expertise in research and development within cardiometabolic diseases and Omega’s proprietary platform technology to develop an epigenomic controller designed to enhance metabolic activity.
- Unlike traditional approaches focused on appetite suppression, the program aims to leverage precision epigenomic control to enhance thermogenesis, a naturally occurring metabolic process that burns calories.
- Under the terms of the agreement, Novo Nordisk will reimburse all R&D costs and has the right to select one target to advance for clinical development. Omega and Flagship’s Pioneering Medicines are eligible to receive up to $532 million in upfront, development and commercial milestone payments, as well as tiered royalties on annual net sales of a licensed product, which will be split equally between the parties.
- Continued to advance and expand OMEGA platform capabilities:
- Presented new preclinical data supporting the breadth of Omega’s platform capabilities, including bidirectional and multiplexed epigenomic control of gene expression in liver inflammation and fibrosis at the American Association for the Study of Liver Diseases’ (AASLD) The Liver Meeting® 2023.
- A HNF4A-targeting epigenomic controller led to a durable increase in HNF4α expression, preferential upregulation of HNF4α P1 promoter isoforms, and reduced key measures of fibrosis both in vitro and in vivo, supporting this development candidate’s potential for the treatment of fibrotic liver disease.
- In preclinical models, liver-specific multiplexed targeting of CXCL9, CXCL10 and CXCL11 via an epigenomic controller led to a significant reduction in T-cell migration, a critical driver of inflammation-induced liver injury, supporting the potential of this approach as a novel treatment for inflammatory liver diseases.
- Presented new preclinical data supporting the breadth of Omega’s platform capabilities, including bidirectional and multiplexed epigenomic control of gene expression in liver inflammation and fibrosis at the American Association for the Study of Liver Diseases’ (AASLD) The Liver Meeting® 2023.
Corporate
- Announced cost reduction and strategic prioritization initiative to maximize near- and long-term value creation opportunities:
- Following a strategic review, the Company has focused its pipeline and reduced overall headcount by approximately 35%. These fiscally disciplined actions are expected to extend the Company’s cash runway into Q1 2025.
- Positions the Company to achieve key clinical data readouts from the monotherapy dose escalation and dose expansion stages of the MYCHELANGELO I clinical trial.
- The Company will prioritize certain preclinical programs and platform efforts:
- Prioritized preclinical programs include OTX-2101 for non-small cell lung cancer (NSCLC), the HNF4A program in liver regeneration, and development of an epigenomic controller for obesity in collaboration with Novo Nordisk.
- Core work on platform biology, epigenomic controllers, and characterization of LNP delivery to the lung and other tissues will continue.
- An updated corporate presentation is available on the Investors section of the Company’s website at https://ir.omegatherapeutics.com/.
Fourth Quarter and Full Year 2023 Financial Results
As of December 31, 2023, the Company had cash, cash equivalents and marketable securities totaling $73.4 million, which is expected to fund operations into Q1 2025.
Research and development (R&D) expenses for the fourth quarter of 2023 were $15.5 million, compared to $26.0 million for the fourth quarter of 2022. R&D expenses for 2023 were $77.2 million compared to $81.2 million in 2022. The $4.0 million decrease in R&D expenses in 2023 compared to 2022 was primarily due to lower external research and manufacturing costs, consulting and professional fees, and lab expenses, partially offset by an increase in personnel-related expenses, including stock-based compensation to support business growth, and facilities and other costs.
General and administrative (G&A) expenses for the fourth quarter of 2023 were $6.2 million, compared to $5.7 million for the fourth quarter of 2022. G&A expenses for 2023 were $26.2 million, compared to $23.7 million in 2022. The $2.5 million increase in G&A expenses in 2023 compared to 2022 was primarily due to higher professional and consulting fees, and facilities and other administrative costs.
Net loss for the fourth quarter of 2023 was $20.2 million, compared to $30.8 million for the fourth quarter of 2022. Net loss for the year ended December 31, 2023, was $97.4 million, compared to a net loss of $102.7 million for the year ended December 31, 2022. The decrease in net loss for 2023 compared to 2022 was primarily due to decreases in R&D expenses.
금년 3월에 발표한 Corporate Presentation을 첨부합니다.
