
(Picture: Frédéric Tangy, PhD, Dr.Sc. Institut Pasteur)
Themis Bioscience Completes €5M Series A Financing – FinSMES 5/21/2011
Themis Bioscience, a Vienna, Austria-based biotechnology start-up developing vaccines to prevent infectious diseases, has completed a €5m Series A financing round.
The round was co-led by Ventech and Crédit Agricole Private Equity.
The company intends to use the funds to advance the pre-‐clinical and clinical development activities for its lead product candidates, a Dengue and a Chikungunya Fever vaccine, which are both based on a novel vaccine vector technology (Themaxyn™) that was initially developed at the Institut Pasteur in Paris.
Founded in 2009 and led by CEO Erich Tauber, Themis Bioscience received seed financing by the academic business incubator INiTS, the austria wirtschaftsservice (aws) and The Austrian Research Promotion Agency (FFG).
VIENNA, Austria I March 02, 2015 I Themis Bioscience (‘Themis’), a biotechnology company developing innovative prophylactic vaccines for emerging tropical infections, and the Institut Pasteur, an international biomedical research center based in Paris (France) today announced the publication of the phase I study results for a recombinant measles-virus-based chikungunya vaccine (MV-CHIK) in The Lancet Infectious Diseases. The study was performed in collaboration with the Department of Clinical Pharmacology at the Medical University of Vienna and the Viral Diseases Branch of the Walter Reed Army Institute of Research (WRAIR) in the USA.
The peer-reviewed article is entitled “Immunogenicity, safety, and tolerability of a recombinant measles-virus-based chikungunya vaccine: a randomised, double-blind, placebo-controlled, active-comparator, first-in-man trial“.
Chikungunya fever is a mosquito-borne viral disease causing symptoms including fever, headache, joint and muscle pain and bleeding of the nose and gums. Importantly, a large number of infected patients suffer from chronic sequela months and years after the acute infection. The chikungunya virus originated in Asia and western and central Africa and rising levels of travel and global warming led to increasing incidences of the disease in temperate zones, thus becoming a global health threat. Since late 2013, more than one million cases have been reported in the Americas and the Caribbean alone, resulting in a significant public health and economic burden.
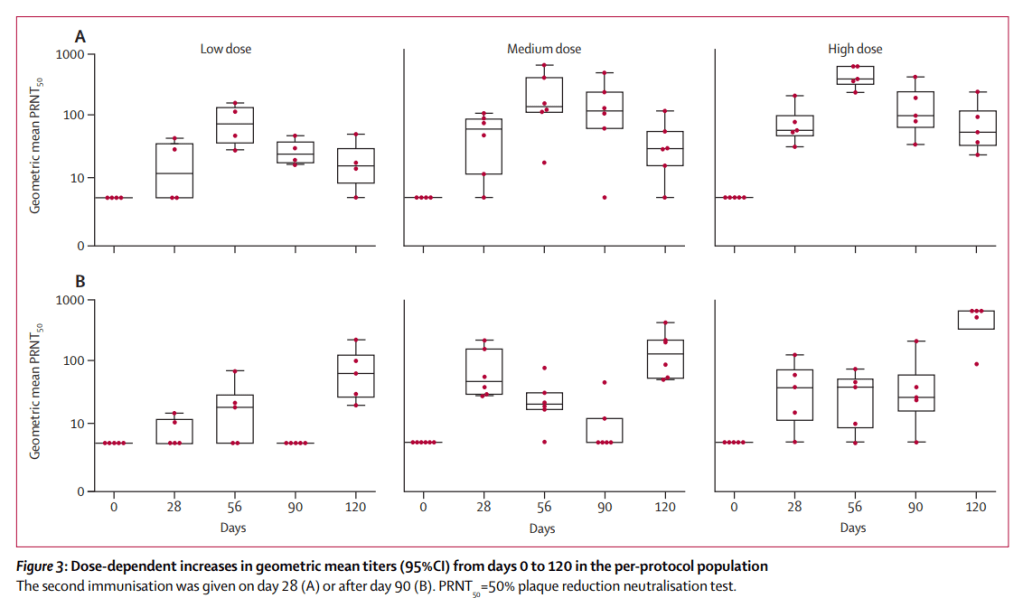
Themis’ recombinant measles-chikungunya vaccine phase I study was conducted between November 2013 and June 2014 with a total of 42 healthy male and female individuals from age 18-45 being randomised into 4 cohorts for this dose escalation study. Subjects were administered one injection with either a low, medium or high dose of the chikungunya vaccine or the active comparator Priorix (standard measles vaccine). The study investigated the immunogenicity, safety and tolerability of the vaccine. In addition, randomized participants received a booster injection on either day 28 or day 90 after the first vaccination.
The candidate vaccine raised concentrations of neutralising antibodies to chikungunya in all dose cohorts after one immunisation, with seroconversion* rates of participants producing anti-chikungunya antibodies of 44% in the low, 92% in the medium, and 90% in the high-dose group. The immunogenicity of the candidate vaccine was not affected by pre-existing anti-measles immunity. The second vaccination resulted in a 100% seroconversion for all participants in the candidate vaccine groups. The candidate vaccine had an overall good safety profile, and while the rate of adverse events increased with vaccine dose and volume, no vaccination-related serious adverse events were recorded.
Dr. Frederic Tangy, head of the Viral Genomics and Vaccination Unit at the Institut Pasteur (Institut Pasteur, CNRS UMR-3569), who developed this vaccine technology, explained: “The measles vaccine has already proven its high efficacy and safety on more than a billion vaccinated individuals during the last 30-40 years. Therefore, this platform offers an excellent safety profile and the clear advantage of a validated and easy manufacturing process. The present result demonstrates that a measles vector can be used in the presence of pre-existing immunity to measles, likely because it is a replicating vector. This gives another great advantage to this vaccine strategy.”
“Recent outbreaks have raised awareness of chikungunya virus worldwide and whilst further work is needed to show safety, tolerability, and ability of the vaccine to protect against live chikungunya virus, our trial data suggest that this novel vaccine is an excellent candidate to help address this urgent medical need”, explains Dr. Erich Tauber, CEO of Themis. “With these promising results we are advancing the chikungunya vaccine programme and aim to move rapidly into phase II studies.”
* seroconversion rate: percentage of participants/patients that produce antibodies
About Themis:
Themis Bioscience GmbH develops prophylactic vaccines with a focus on emerging tropical infectious diseases and has initial vaccine candidates currently in development for chikungunya and dengue fever. The company has exclusive, worldwide licenses for chikungunya- and dengue vaccines, based on the innovative and fully patent-protected measles virus vaccine vector platform from the Institut Pasteur in Paris. This platform underpins Themis’ growing pipeline of vaccines. Themis and Institut Pasteur are actively collaborating on additional targets. Themis was founded by experienced vaccine experts in September 2009 and is based in Vienna. For more information, visit the website: http://www.themisbio.com
Themis Bioscience’s Raises up to EUR 10 Million in Series B – Fierce Biotech 5/6/2015
Themis Bioscience (‘Themis’), a biotechnology company developing innovative prophylactic vaccines for emerging tropical infections, announced today the first closing of EUR 7 Million in a Series B financing of up to EUR 10 Million, led by new investor Wellington Partners. Existing investors Ventech and Omnes Capital (formerly Crédit Agricole Private Equity) also participated in the round. Dr Regina Hodits, General Partner at Wellington Partners will join Themis’ Board.
With their Chikungunya fever vaccine candidate demonstrating good immunogenicity, safety and tolerability in a Phase 1 clinical trial (Results published in The Lancet Infectious Diseases, March 2015), Themis plans to progress this lead product candidate into clinical phase II trials. In parallel the company will advance its other promising development pipeline in collaboration with the Institut Pasteur in Paris, originators of the measles vector platform licensed to Themis.
Themis also announced the new structure of its Board with Dr Gerd Zettlmeissl being named Chairman of the Board. Dr Zettlmeissl spent more than 20 years in executive positions in the international pharmaceutical and vaccine biotech industry. From 2005 until May 2011 he served as CEO of Intercell AG. Experienced biotech and vaccine industry expert Dr Jean-Paul Prieels will join as a new member the expanded board. He served as Senior Vice President of R&D at GlaxoSmithKline Biologicals until January 2011, led GSK’s global vaccine R&D development activities and was Head of Research for GSK Vaccines.
Dr Erich Tauber, CEO of Themis stated: “With the new funds, we are planning to move our Chikungunya vaccine candidate quickly into a Phase 2 clinical trial and also achieve important milestones for the other vaccine candidates in our preclinical development pipeline. We are very pleased to have Wellington Partners leading this financing round and I would like to welcome Dr Regina Hodits and Dr Jean-Paul Prieels to the Board. I am sure that Themis will profit from their scientific and industrial expertise in supporting our goals to establish new partnerships within the pharmaceutical industry and to drive our product pipeline towards commercialisation.”
Dr Regina Hodits, General Partner at Wellington Partners, commented: “With global warming and increased travel activities, tropical diseases like Chikungunya, Dengue fever, and other viral diseases are becoming a serious threat to global health. Based on a proven measles vaccine platform, Themis’ portfolio of vaccine candidates represent an attractive investment opportunity for Wellington, and they have the potential to address urgent unmet medical needs.”
In 2011, Themis raised EUR 5 Million in a series A financing following a seed financing round from Austria Wirtschaftsservice (AWS) in 2009, and other substantial financial contributions from Austrian national funding agencies like FFG and Inits.
Themis nabs €10M series C for chikungunya, Zika push – Fierce Biotech 1/9/2018
About a year after a €10 million series B, Austrian vaccine company Themis has secured a series C in the same amount led by new investor Global Health Investment Fund (GHIF).
The money will again be used to advance a chikungunya vaccine, which is being tested in three phase 2 trials in central Europe, Puerto Rico and U.S. mainland, a Zika candidate that entered human testing last April, as well as other preclinical assets against RSV and norovirus. These vaccines are based on Themis’ proprietary Themaxyn platform developed at Institut Pasteur, which uses a measles vaccine as a vector to carry antigen-encoding genes.
Themis recently reported positive interim results from the European trial on its chikungunya vaccine, the most advanced program in its pipeline. The vaccine induced neutralizing antibodies in all treatment groups 56 days after first immunization, and the seroconversion rate reached 95% after two doses.
The European trial will have final readouts midyear, but CEO Erich Tauber, Ph.D., told FierceVaccines that the U.S. and Puerto Rico trials were delayed by hurricanes Harvey and Maria. The program also received £3 million worth of funding from the U.K.’s National Institute for Biological Standards and Control to develop a monkey challenge model and to conduct a small phase 1 in the country.
New investor GHIF led the round because it sees “tremendous potential in Themis’ technology platform” and is “impressed with Themis’ ability to navigate complex clinical, regulatory and manufacturing issues,” commented GHIF partner Glenn Rockman, who has joined Themis’ board.
While at J.P. Morgan’s investment banking division, Rockman worked with the Bill & Melinda Gates Foundation to build GHIF. The fund is focused on late-stage projects in drugs, vaccines and diagnostics for diseases that burden low-income populations. It has supported projects in malaria, tuberculosis, HIV/AIDS, cholera and preventable causes of maternal and infant mortality.
No vaccine is available for either chikungunya or Zika, both mosquito-borne viruses. In chikungunya, Themis is notably vying against PaxVax, which in-licensed its candidate from the NIH, and India’s Bharat Biotech and secretive Moderna are working on their phase 1 programs. More candidates are competing in Zika, including one from Inovio, a U.S. Army-developed shot Sanofi recently walked away from, and one from a Valneva-Emergent BioSolutions partnership, among others.
The Coalition for Epidemic Preparedness Innovations (CEPI), the high-profile public-private vaccine initiative launched in 2017, has signed its first company agreement, granting Themis an investment of up to $37.5 million to develop new vaccines against Lassa fever and MERS.
The grant spans a five-year period and will support Themis through phase 2 testing, providing safety and immunological data plus the manufacturing of investigational supplies for efficacy trials or emergency deployment during outbreaks.
Discoveries made by Institut Pasteur and the Paul Ehrlich Institut are set to become the basis for Themis’ Lassa and MERS candidates, respectively. Those two research institutions have identified antigens for inclusion in vaccine compositions and have demonstrated proof of concept in animal studies, Themis CEO Erich Tauber told FierceVaccines.
Themis will apply its measles vector platform—which it exclusively licensed from Institut Pasteur—to design the vaccines. The platform has been used in the company’s lead program for Chikungunya, which is in phase 2 trials in 600 patients in the U.S. and Europe. Its Zika candidate also uses the platform and has entered human testing, while other assets against norovirus, RSV and CMV are in preclinical stages.
“The fact that Themis has developed a versatile technology platform for the discovery, development and production of vaccine approaches is very attractive,” CEPI spokeswoman Rachel Grant told FierceVaccines. “This means as well as focusing on MERS and Lassa we hope this technology will have value beyond those specific diseases. ”
No additional financial details were disclosed, but given CEPI’s founding principle of equitable access, Grant said the agreement contains provisions that support providing vaccines at affordable costs to people in need.
CEPI focuses on epidemic vaccine development, especially where there’s unfavorable market incentives but potentially big public health benefits. The idea is to fund promising vaccine candidates so that they’re available immediately when an outbreak begins.
Officially launched in 2017 by governments and nonprofits such as the Bill & Melinda Gates Foundation and Wellcome Trust, the group has also attracted major vaccine makers including GlaxoSmithKline, Merck, Johnson & Johnson, Pfizer, Sanofi and Takeda. It has so far collected $630 million of its $1 billion target funding. The European Commission has also promised a contribution of €250 million that will support relevant projects through its own mechanisms.
To start, CEPI selected Lassa, MERS and Nipah as initial diseases to target, none of which have approved vaccines. The coalition actually aims to develop two promising vaccine candidates against each of these diseases, and Grant said CEPI is going through intensive technical and legal due diligence with a number of companies to finalize additional agreements over the coming months.
For a second project that will identify platforms for rapid vaccine development against unknown pathogens, Grant said a call for proposal has received 35 high-caliber applications. They’re currently being shortlisted through an external peer review process, and CEPI’s scientific advisory committee will reach a conclusion by the end of June.
The grant comes as Nigeria suffers an unprecedented Lassa fever outbreak. The overall fatality rate is 1%, but for this year, it has reached 22% among confirmed and probable cases in the current Nigerian outbreak, the WHO reported.
MERS, first identified in 2012, causes severe respiratory illness, and it resulted in 186 cases and 36 deaths during an outbreak in South Korea in 2015.
A partnership between Inovio and South Korea’s GeneOne, with help from the Walter Reed Army Institute of Research, currently has the most advanced MERS vaccine program in phase 1 testing.
As for Lassa, according to a comprehensive summary by CEPI, no vaccine has progressed out of preclinical stages. Inovio, for one, is working on a candidate with the U.S. Army Medical Research Institute for Infectious Diseases.
Chikungunya vaccine developer Themis bags $21m – European Biotechnology 6/5/2019
Under Themis’ second partnering agreement with the Coalition for Epidemic Preparedness Innovations (CEPI), the Vienna-based company is eglible to receive up to $21m to push Phase III testing of its Chikungunya vaccine candidate, MV-CHIK. CEPI’s Chikungunya-vaccine development mandate was launched in 2019 with support from the European Commission’s Horizon 2020 programme.
In Phase II trails, Themis’ live-attenuated, measles-vectored chikungunya vaccine (MV-CHIK), which has FDA fast track and EMA PRIME status, showed good safety and tolerability as well as immunogenicity. Themis announced that the non-dilutive funding will provide a significant portion of the capital required for Themis’ Phase III clinical trial of MV-CHIK expected to start this year. The pivotal multi-center Phase III trial will be launched in Europe, US and the Americas and will also test a single-shot regimen.
The World Health Organization (WHO) has highlighted Chikungunya, which causes arthritis-like symptoms, as a major public health risk. The disease was first identified in Tanzania in 1952, with sporadic outbreaks of the disease reported subsequently across Africa and Asia. In 2004 the disease began to spread quickly, causing large-scale outbreaks around the world. Climate change is set to further amplify the threat posed by Chikungunya. As the climate warms, more areas across the world will become habitable for the mosquito vectors that transmit the virus, thereby increasing the size of the human population at risk of infection. In 2007, for example, an outbreak of Chikungunya virus infections was declared for the first time in Europe, with more than 200 human cases reported in Italy.
Since the re-emergence of the virus, the total number of cases has been estimated at over 3.4 million in 43 countries.Chikungunya is spread by the bites of infected female Aedes mosquitoes and causes fever, severe joint pain, muscle pain, headache, nausea, fatigue and rash. Joint pain is often debilitating and can persist for weeks to years.
Themis’ first partnership with CEPI, announced in March 2018, provided up to $37m in funding to support vaccine development and manufacturing for Lassa fever and MERS.
Merck & Co. Partners with Themis on Measles Virus Vector-Based Vaccines – GEN Edge 8/22/2019
Merck & Co. will partner with Themis Bioscience to develop vaccine candidates based on Themis’ measles virus vector-based platform, through the Austrian biotech’s first-ever collaboration with a major biopharma.
Themis says the platform, which it licenses from the Institut Pasteur in Paris, can incorporate large recombinant genes coding for selected antigens into its genome. Vaccines developed through the platform are designed to deliver multiple selected antigens—such as full-length proteins or virus-like particles—directly to macrophages and dendritic cells, thus triggering a specific immune response to the selected antigens.
The companies have committed to developing vaccine candidates against an undisclosed disease target. Speaking with GEN, Themis CEO Erich Tauber, MD, would not disclose what indications the companies are focusing on.
“What I can say is the measles virus vector technology allows to exploit infectious diseases and cancer indications, and when it comes to infectious diseases, we use the measles virus to bring our specific antigens into the body. Those might be difficult to express in normal cell systems,” Tauber said.
“Another advantage is we can use exactly the same manufacturing process for each new vaccine target. And when it comes to cancer, the measles virus itself has strong oncolytic activity,” Tauber said, such as mediating tumor cell lysis, T cell activation, and specific tumor cell targeting. “The measles virus can kill cancer cells. And we enhance this activity by putting in specific therapy enhancing proteins like immune modulators.”
Merck has agreed to make an unspecified equity investment in Vienna-based Themis under the companies’ collaboration and exclusive license agreement, which Themis said could generate for it more than $200 million.
In addition to the equity investment, Merck agreed to provide Themis an unspecified amount of research funding, as well as up to approximately $200 million in payments tied to achieving development and sales milestones, plus royalties on approved products from the collaboration.
“We continue to evaluate technologies with the potential to deliver novel vaccine candidates,” Daria Hazuda, PhD, CSO, Exploratory Science Center and vp of infectious diseases and vaccine discovery at Merck, said in a statement. “We look forward to collaborating with the scientists at Themis.”
Surging vaccines sales
Merck credits human vaccines, along with cancer treatments led by the blockbuster immunotherapy Keytruda® (pembrolizumab), with its most recent positive quarterly results.
During the second quarter, Merck said, overall company sales rose 12% year-over-year to $11.760 billion—or 15% excluding the effect of foreign exchange rates. The biopharma giant finished Q2 with $2.670 in GAAP net income, up 56% from $1.707 billion in the second quarter of 2018.
However, human health vaccines sales zoomed 33% year-over-year, to $2.0 billion, or 36% when excluding currency impacts. Two vaccines led Merck’s sales surge: PROQUAD (Measles, Mumps, Rubella and Varicella Virus Vaccine Live) and VARIVAX (Varicella Virus Vaccine Live), a vaccine to help prevent chickenpox, saw their combined sales jump 58% in Q2, to $675 million from $426 million in the year-ago quarter. Merck cited higher demand, including from private-sector buyers, and U.S. pricing, as well as government tenders in Latin America and higher demand in Europe.
Merck also reported a 46% quarterly sales jump (50% excluding currency impacts)—to $886 million from $608 million in Q2 2018—for the tandem of GARDASIL [Human Papillomavirus Quadrivalent (Types 6, 11, 16, and 18) Vaccine, Recombinant] and GARDASIL 9, vaccines indicated to treat some cancers and other diseases caused by HPV. Merck cited primarily public sector buying patterns, U.S. demand and pricing, and the ongoing commercial launch in China, as well as higher demand in Europe, driven primarily by increased vaccination rates for both boys and girls.
Expanding pipeline range
Merck’s collaboration with Themis is likely to expand the range of indications for vaccine candidates in the pipeline of Themis, which has focused most in developing vaccines against infectious diseases, but also has several preclinical immuno-oncology programs.
In October 2018, Themis signed an exclusive worldwide license agreement of undisclosed value with Max-Planck-Innovation GmbH, the technology transfer agency of the Max Planck Society, to develop, manufacture, and commercialize therapies based on an oncolytic measles virus platform jointly developed by the Eberhard-Karls-University Tübingen and the Max Planck Institute for Biochemistry.
Themis’ most advanced pipeline program is an unpartnered chikungunya vaccine candidate MV-CHIK, which Tauber said remains on track for a Phase III trial set to begin later this year. The pivotal multi-center trial will test a single-shot regimen, with patients to be dosed at centers in Europe, the U.S., and the Americas.
In June, Themis was awarded up to $21 million in non-dilutive capital toward development of the chikungunya vaccine by the Coalition for Epidemic Preparedness Innovations (CEPI), part of CEPI’s third call for proposals with support from the European Union’s (EU’s) Horizon 2020 research funding program under grant agreement No. 857934. The award is intended to accelerate regulatory approval of the chikungunya vaccine and ensure that at-risk populations have access to the vaccine by funding a “significant” portion of the capital needed for the Phase III trial, Themis said at the time.
Themis once planned to fund the Phase III trial through an up-to-€55.3 million ($61.3 million) initial public offering on Euronext Amsterdam, but postponed the IPO in November 2018, citing adverse market conditions.
The chikungunya candidate is also under study in a Phase II trial (NCT03807843) designed to assess the vaccine’s safety and immunogenicity in adults with a history of chikungunya infection; and another Phase II trial (NCT03635086) designed to investigate the immunogenicity, safety, and tolerability of MV-CHIK as well as the long-term durability of anti-chikungunya antibody response after administration of different dose levels of the vaccine in three different formulations.
Last year, CEPI awarded Themis up to $37.5 million toward developing vaccines for Lassa fever and Middle East respiratory syndrome (MERS).
Merck inks Themis buyout to join COVID-19 vaccine race – Fierce Biotech 5/26/2020
Merck has struck a deal to buy Themis to accelerate the development of a COVID-19 vaccine. The takeover will see Merck, a latecomer to the response to SARS-CoV-2, apply its vaccine capabilities to a candidate based on Themis’ measles vector platform that is set to enter the clinic this year.
Themis is developing a pipeline of vaccines based on a measles virus vector platform it licensed from Institut Pasteur. By engineering the virus to express different antigens, Themis aims to use the same vector and manufacturing system to develop vaccines that induce protection against a wide range of infectious diseases, including COVID-19.
“Together with Institut Pasteur, we have worked on very closely related viruses like SARS and MERS [and] demonstrated the platform is very useful in eliciting an immune response,” Themis CEO Erich Tauber said. “We started [SARS-CoV-2] vector design in February. We have started in vivo models … and are now preparing for clinical trials.”
Merck is now set to apply its vaccine capabilities to the program. The Big Pharma has a major human vaccine operation, which generated sales of $8.4 billion last year, but it stayed on the sidelines in the early days of the pandemic as peers such as AstraZeneca, Pfizer and Sanofi placed bets on COVID-19 vaccine candidates.
News of a change in strategy came late in April when Merck said it was talking to “multiple groups” about three viral vector platforms. The talks manifested in an agreement to buy Themis, a privately owned Austrian biotech, for an undisclosed sum. In selecting Themis as a key plank of its COVID-19 strategy, Merck has indicated it thinks the biotech’s vaccine can clear a high bar.
“The task before us is one that requires a vaccine that will be quite stimulatory and that will yield neutralizing antibodies ideally with a single immunization. Of course, it must first be safe because you’re talking about a vaccine that would in principle be given to much of the world’s population,” Roger Perlmutter, president of Merck Research Laboratories, told investors last month.
Themis, as part of a consortium featuring Institut Pasteur, partnered with the Coalition for Epidemic Preparedness Innovations (CEPI) to develop a COVID-19 vaccine in mid-March. Earlier this month, Themis disclosed a deal with service provider ABL Europe covering the production of the vaccine in France.
Merck, which plans to start testing the vaccine in humans this year, has previously said it is trying to identify internal resources and contract manufacturers that can enable it to produce 1 billion doses of a COVID-19 vaccine and plans to make Themis’ shot at sites in the U.S. and Europe. The ability of Merck to bring such scale to bear factored into Themis’ decision to sell up.
“The limiting step for everybody will be manufacturability and manufacturing capacity. Merck brings an enormous skill level, expertise and capacity in terms of manufacturing technology. They’re using very similar technology already. They have been manufacturing measles vaccines for 60 years or so,” Tauber said.
Merck bought into the concept behind Themis’ platform last summer when it tasked the biotech with developing vaccines against an undisclosed target and invested in its series C round. Now, Merck has decided to acquire its partner outright.
The takeover will give Merck ownership of the platform, which Themis thinks has immuno-oncology applications, and a pipeline led by a phase 3-ready chikungunya vaccine candidate. Merck also sees the acquisition as a way to “ build a pandemic preparedness capability” against future threats.
For now though, COVID-19 is the focus. Merck and CEPI have entered into a memorandum of understanding about the need to make the vaccine “accessible to those who need it, including low-income, middle-income and high-income countries, based on the medical need.” Tauber raised Merck’s approach to vaccine access in explaining why it is the right partner, citing the Big Pharma’s work on Ebola as evidence that it is “very enthusiastic about global supply of vaccines.”
After Merck & Co. got off to a late start in the COVID-19 vaccine race and made an early exit, the drug giant is in talks to aid the global vaccine manufacturing effort.
The drugmaker is “actively involved” in discussions with governments, health agencies and other pharmaceutical companies to “identify the areas of pandemic response where we can play a role, including potential support for production of authorized vaccines,” a spokesman said via email.
News of the talks comes about two weeks after Merck abandoned both its coronavirus vaccine candidates—one it acquired through its Themis buyout and the other it was studying in partnership with IAVI. Merck said the two shots had produced immune responses weaker than those prompted by natural infections as well as by other COVID-19 vaccines.
Still, the company believes it has an “important responsibility to contribute to the pandemic response,” the spokesman said, and remains “at the ready to do so.”
While Merck hasn’t indicated which companies it could help with production, there has been industry talk about a potential tie-up with Novavax. After the vaccine biotech last month presented positive phase 3 data on its candidate, Evercore ISI analyst Josh Schimmer said he suspected Merck might “step up” as Novavax’s manufacturing partner.
Novavax CEO Stan Erck then told CNBC’s Meg Tirrell that Merck “could be a good partner for us as they don’t have a competing product.” He also named GSK as a company with those capabilities. At the time, Merck told Tirrell it was focused on therapeutics.
Meanwhile, Merck has two coronavirus therapeutics in development—MK-4482 and MK-7710—and the company believes it can make a “meaningful contribution” to the fight against the pandemic by focusing its resources on those candidates, its spokesman said.
Last summer, as COVID-19 vaccine programs raced forward, Merck CEO Ken Frazier said the hype about vaccines launching in late 2020 was doing a “grave disservice” to the pandemic fight. Vaccines previously took years to develop, he pointed out, and Merck itself was responsible for many of them.
He wasn’t alone. Merck and other major vaccine players were taking a slower, time-tested approach, experts said, but their vaccines could end up reaching more people worldwide than more revolutionary shots would. Things didn’t turn out that way. Pfizer, Moderna, AstraZeneca and other programs are now either rolling out or nearing rollouts, while several leading vaccine giants have either exited the field or faced R&D setbacks.
If Merck does strike a manufacturing deal with a COVID-19 vaccine player, it won’t be the first company to do so. After an R&D setback on its GSK-partnered vaccine, Sanofi last month said it would produce 100 million doses of the Pfizer-BioNTech mRNA vaccine for Europe.
The Pfizer-BioNTech team has also enlisted Swiss drugmaker Novartis in its global push to produce billions of doses. In a deal unveiled in late January, Novartis said it would allow BioNTech access to its site in Stein, Switzerland. Manufacturing there will start next quarter, and doses will be ready from the site by the third quarter.
Also this week, Teva said it was in talks to help with COVID-19 vaccine production. The company has sites in Israel, Europe and the U.S. that could be used in the global effort, CEO Kåre Schultz said, according to The Wall Street Journal.
