
안녕하세요 보스턴 임박사입니다.
이제 완연한 여름이네요. 뉴잉글랜드에도 반팔에 반바지 차림의 사람들이 거리에 즐비합니다. 저는 몇달 전부터 보스턴으로 출근을 하는데 오랜동안 케임브리지로 출근을 할 때와는 또 사뭇 다른 느낌이 나는군요. 제가 근무하는 보스턴은 레드삭스의 전용구장인 펜웨이파크가 있는 곳이어서 항상 야구펜들로 북적입니다. 케임브리지에서는 주로 학생들, 연구원들, 교수나 바이오텍 제약회사 사람들만 즐비했는데 보스턴에 오니까 다양한 일반인 (?)을 만날 수 있는 것 같습니다.
이번주에 보스턴에서는 BIO 행사가 있었습니다. 덕분에 바이오 행사에 참여한 분들을 몇분 만났는데 VC 한분을 통해서 새로운 모델의 회사가 있다는 얘기를 듣게 되어서 이 회사에 대해 얘기를 좀 해 보고자 합니다.
오늘 소개하고 싶은 회사는 Formation Bio 라는 회사입니다. 이 회사는 2016년에 Yale 출신으로 Oxford에서 Computational Biology 박사인 Benjamin Liu에 의해 세워진 회사로 바이오텍이나 빅파마에서 신약을 사온 후 AI를 활용해서 임상을 촉진시키는 모델의 회사입니다. 작년 중순에 $372M 규모의 시리즈 D를 받았습니다.
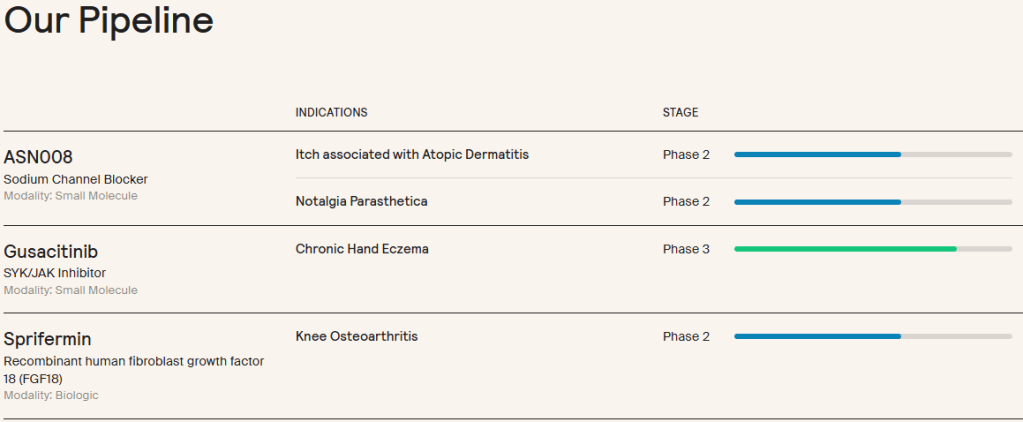
“Formation, which was formerly known as TrialSpark, said it plans to deploy this capital to continue to acquire and in-license clinical-stage assets from its biotech and pharma partners. The company currently lists three candidates in its clinical pipeline, including a SYK/JAK inhibitor called gusacitinib in phase 3 development for chronic hand eczema. There’s also a sodium channel blocker called ASN008 in phase 2 trials for notalgia paresthetica and itch associated with atopic dermatitis as well as an FGF18 drug called sprifermin in phase 2 for knee osteoarthritis.
Both gusacitinib and ASN008 were bought from Asana BioSciences in 2022, while sprifermin was licensed from Merck KGaA.”
현재 진행 중인 임상 파이프라인은 3개의 약물이 있습니다. 두개의 약물은 2022년에 Asana BioSciences에서 인수한 약물이고 나머지 하나는 Merck KGaA에서 사온 약물입니다.
그리고 최근에 4명의 EIR (Entrepreneur-In-Residency)를 채용해서 신약 파이프라인을 증대시키려는 계획을 발표했습니다. 이 중에 제가 아는 한국계 분도 계시네요. 이 네분의 EIR을 통해서 다양한 질환군에 대한 BD를 하려는 것으로 보입니다.
“Formation Bio, an AI-driven pharmaceutical company focused on accelerating drug development, today announced the appointment of its first class of Entrepreneurs in Residence (EIRs): Kia Motesharei, Ph.D., Minji Kim Ph.D, MBA, John Taylor, M.S., and Anthony S. Walsh, D.Phil, These seasoned dealmakers will work closely with Chief Business Officer David Steinberg to expand the company’s business development capabilities and further its mission to bring high-potential treatments to patients faster.”
Ben Liu의 창업스토리는 3년전에 Forbes에서 다룬 적이 있습니다. 처음에는 자신이 개발한 신약을 빅파마에 팔려고 하다가 오히려 신약은 많지만 임상이 어렵다는 얘기를 듣고 창업을 결심하게 되었다는 이야기가 흥미롭게 다가옵니다.
Immigrant Ben Liu And TrialSpark Revolutionizing Clinical Trials – Forbes 08/10/2022
A Letdown That Turned Into An Epiphany: “I remember naively going to pharma executives when we discovered these drugs and said, ‘Aren’t you guys as excited as we are?’ because I thought that discovering the drugs is the biggest challenge,” said Liu. “The pharma executives said, ‘I hate to burst your bubble, but we actually, every quarter, already have enough good discovered drugs to develop. But a single clinical trial drug development process can cost tens of millions, if not hundreds of millions of dollars, and that’s really where our bottleneck is. We could stop our research efforts today and we would already have more than enough good drugs discovered than we could possibly move forward with.’”
This proved to be the genesis of TrialSpark. “What motivated us to start the company was understanding that if we already live in a world where there is an abundance of good drug candidates, and no one truly knows what’s going to work until you run a clinical trial, the one competitive advantage you want to have as a next-generation pharma company is to run trials cheaper and faster,” said Liu. “We all saw this recently with the Covid-19 vaccines. They were discovered very quickly, but the biggest hurdle to bringing the vaccines to the public was the time and cost of the trials.”
Formation Bio의 전신인 TrialSpark는 Covid-19이 한창인 2021년 9월에 $156M의 시리즈 C를 받았습니다. 모두가 아무 것도 하지 못할 때 어떤 사람은 끊임없이 이렇게 큰 사업을 기획하고 있습니다.
TrialSpark raises $156MM Series C Funding led by Sam Altman and Lachy Groom – PR Newswire 09/30/2021
TrialSpark is building a full stack pharma company that can develop drugs in-house faster and more efficiently than traditional pharma companies, driven by the belief that every day saved in the clinical trial process is one day sooner that a patient can access a life changing treatment.
Our Vision for AI in Pharma – Formation Bio Blog by Benjamin Liu 05/01/2024
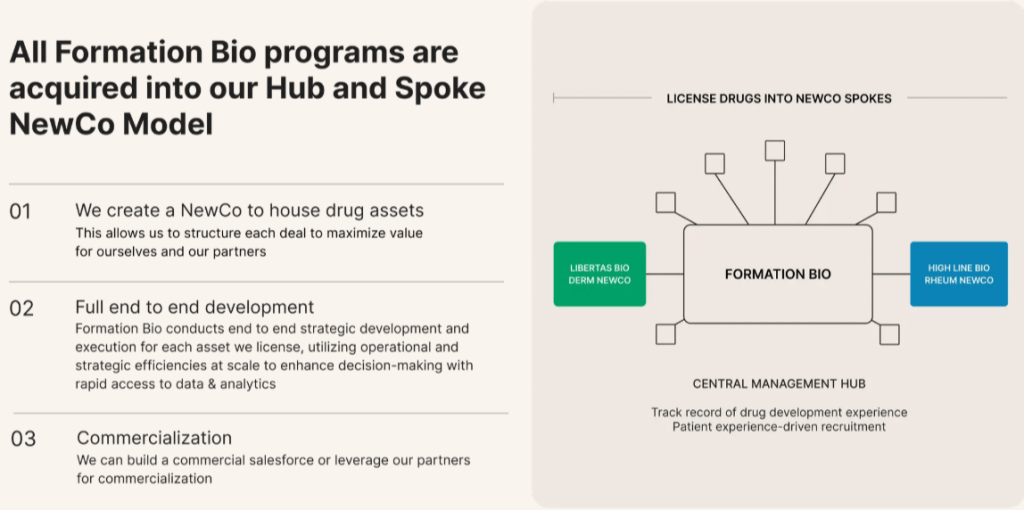
We structure each NewCo bespoke to partner preferences — which could include equity, upside, or royalties — and provide the capital and capabilities needed to advance the asset. We win when our partners win – we can sell the drug to pharma post Phase 2 readout, find a pharma partner after PoC is achieved, continue to advance the program ourselves, and even commercialize if needed.
Where our approach is differentiated is our ability to run drug development and clinical trials more efficiently. As a tech and AI-native company, we’ve built our own, in-house, tech driven CRO, with all the Quality and Drug Development systems, processes, and expertise needed to run clinical trials. We’ve built proprietary technology and data infrastructure that not only allows us to run trials more efficiently but also sets us up to uniquely capitalize on building and training AI models.
We are also working on an AI R&D Scientist that can provide decision support and eventually steer higher quality R&D decisions.
AI R&D 과학자 – 어떻게 생각하시나요? AI에 대한 다양한 시도가 있는데 연구단계 뿐만 아니라 임상 단계에서도 AI를 활용한 좋은 방법이 시행된다는 것이 흥미롭습니다. 저도 이 부분에 대해 아직 많이 앎이 부족한데 이번 기회로 공부를 좀 해 보려고 합니다.
