안녕하세요 Boston 임박사입니다.
제가 관심을 가지는 분야는 Genetic Medicine Companies (유전자 치료제 회사) 인데요 그 중에서 GV (Google Ventures)에서 초기투자한 Verve Therapeutics에 대한 좋은 News 기사가 있어서 이에 대해 번역글을 올리려고 합니다.
Verve Therapeutics: How To Build A Gene Editing Biotech Life Science Leader (3 Jan 2023)
2016년 Sekar Kathiresan은 미국심장학회 (AHA)에서 주최한 연구비 경쟁 Program에 참여했다. “대담한 아이디어 (One Brave Idea)”라고 불린 경쟁 Program은 심장질환을 치유할 수 있는 Idea 중 최고의 대담한 Idea를 심사해서 당선된 최종 1명에게 $75M (900억원)을 수여하는 Program으로서 미국심장학회, Google의 Verily와 Astra Zeneca가 공동으로 출자한다. Harvard 의대 교수이자 Broad 연구소 인간유전연구실장이었던 Kathiresan은 LDL Cholesterol (즉, “나쁜” Cholesterol)을 한번의 치료만으로 영구적으로 낮출 수 있는 Idea를 제출했다. 그는 수상하지 못했다.
하지만 그는 단념하지 않았고 이 치료법이 심장마비를 크게 감소시킬 수 있는 해답이라고 강하게 확신했고 2018년 대학을 떠나 자신의 회사를 설립했는데 그 회사가 Verve Therapeutics이다. Massachusetts주 Cambridge 소재의 이 회사는 2021년에 기업공개 (IPO)를 하였고 이미 후보물질 VERVE-101의 최초의 임상실험을 시작했는데 이 약물은 유전질환인 가족성 고 Cholesterol 혈증 (HFH) 환자를 대상으로 유전자 편집치료를 하는 약물이다. 기자는 작년 10월에 Kathiresan 으로 부터 회사를 시작하는데 필요한 투자유치, 인재 확보에서 부터 새로운 염기 편집 기술을 임상실험에 도달하기까지 4년도 채 안 걸린 이야기를 들을 수 있었고 이 약물이 승인을 받고 상용화되기까지 넘어야 할 어려움에 대해 배울 수 있었다.
교수에서 창업자로
AHA 연구비를 받을 수 없게된 사실을 알게된 후 Kathiresan은 2년간 Venture Capital 투자 유치, 인력 충원 및 지적재산권 확보를 통해 치료제를 개발하는데 필요한 모든 노력을 쏟았다. Alphabet (Google)의 Google Ventures (GV)가 결정적인 초기 후원자였고 근 2년간 매주 금요일 Group Meeting 을 하면서 회사를 성공적으로 Incubation 해 주었다. Broad 연구소의 Chief Data Officer 임원이자 GV의 Venture Partner인 Anthony Philippakis가 AHA연구비 지원할 때에도 함께 일했는데 이 두사람은 Broad 연구소에서 현재 Vertex Pharmaceuticals CSO 임원으로 있는 David Altschuler 교수의 지도하에 수련을 받았다. AHA 최종우승자가 결정된 후 brainstorming을 통해 Kathiresan과 Philippakis는 회사 Idea를 GV에 제출하기로 합의했다. 2016년에 GV와 연결이 되었고 그들은 우리를 정말 많이 도와주었어요.”라고 Kathiresan은 말했다. “그들은 투자 Network와 함께 회사를 설립할 수 있는 인적자원을 제공했습니다.” GV의 생명과학부분을 이끌고 있는 무한책임투자자인 Krishna Eshwant는 Kathiresan을 “위대한 팀을 끌어들이는 인간 자석”이라고 GV Website에서 Verve사의 IPO를 축하하는 기사에서 인용했다. GV의 후원으로 Kathiresan은 자신의 전문 Network와 신약개발, 유전자편집, 안전성 평가, 사업개발, 지적재산 기술을 가진 다양한 동료들을 Verve 공동창업자로 확보했다.
2018년 Verve Therapeutics는 GV 주도하에 ARCH Venture Partners, F-Prime Capital, Biomatics Capital등과 함께 Series A Funding을 마쳤다. 당해 이사회에서 CEO 역할을 할 사람이 필요하다는 것이 분명해 졌다. 몇사람의 이름이 오고 갔다. 이 회사는 Kathiresan의 Idea에 기초했고 Kathiresan이 지난 2년간 회사가 어떻게 가야할지에 대한 Vision을 만들어냈다. 하지만 “저는 Business 경험이 없었고 이사회가 저를 그 역할에 맡겨줄지 알 수 없었죠. 하지만 CEO 역할에 관심이 있어서 제안을 했습니다.”라고 Kathiresan은 말했다. 이사회는 이 제안을 받아들였고 Kathiresan은 CEO가 되었다.
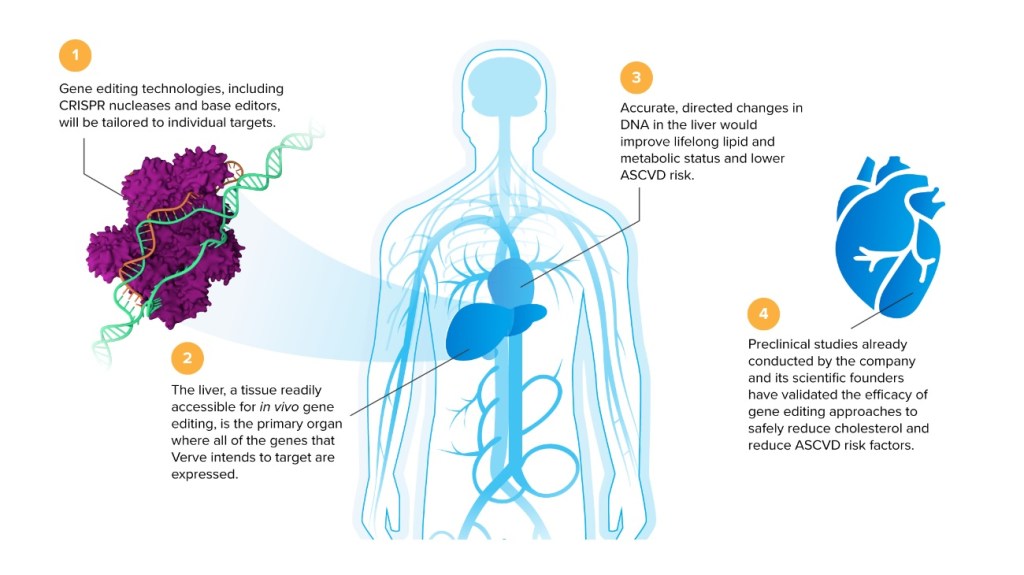
CEO로서 Kathiresan은 과거 연구경험을 통해 심장이 건강하게 하려면 간에서 어떤 유전자를 차단해야 하는지 이미 알고 있었다. 그와 연구진은 유전자 편집기술을 이용해 이들 유전자를 차단해야 한다고 생각했다. 당시 어떤 유전자 편집기술을 사용하느냐는 그다지 중요하지 않았고 우리는 그냥 가장 좋은 기술을 찾고자 했어요.”라고 Kathiresan은 말했다. Verve는 당시로서 두가지 유전자 편집 기술을 활용할 수 있었던 분자 가위로 DNA를 자르는 “표준 CRISPR-Cas9″기술을 Broad 연구소와 Editas medicine에서 확보하고 두번째 기술인 보다 새로운 “염기 편집” 방식을 Beam Therapeutics로 부터 License-In 함으로써 확보할 수 있었다. “당시 우리는 두가지 기술을 가지고 간에 있는 첫번째 표적인 PCSK9을 차단할 수 있는 능력을 비교했습니다.”라고 Kathiresan은 말했다. “세포실험, 쥐실험 및 원숭이 (NHP) 실험으로 비교를 했고 비교한 결과 처음 두가지 약물 후보군에는 염기 편집기술을 사용하는 것이 옳다는 것을 확신할 수 있었습니다.”
또하나 Kathiresan과 Verve 경영진이 2018년에 해야할 초기 선택은 – COVID-19 출현과 mRNA Vaccine 개발을 통해 입증된 – 약물을 간으로 전달하는데에 Virus를 이용할지 LNP (Lipid Nanoparticle)을 이용할지를 결정하는 것이었다. “우리는 Virus보다는 LNP를 사용하기로 결정을 했는데 Virus가 많은 환자군을 치료하기에는 안전성 측면을 염려했다.”라고 말했다. “그리고 원자재 가격 측면에서 대량생산에 미치는 영향도 고려했다.” 몇년 후 COVID-19 mRNA Vaccine이 나왔고 LNP에 내포된 mRNA는 대량생산에서 가격 경쟁력이 있음이 입증되었다. Verve의 선도물질은 mRNA 편집자와 어느 위치를 편집할지 알려주는 guide RNA (gRNA)로 구성된다고 Kathiresan은설명했다. 이들 모두는 핵산물질이고 LNP에 함께 들어가서 혈관에 60-90분간 주입된다. “대량생산이 가능하다는 자신감을 얻었고 대량생산을 가로막는 원가문제는 이론적으로 문제가 되지 않았다. 약물은 매우 가격 경쟁력있게 생산할 수 있다.
Verve는 현재 어떤 공정을 내부에서 생산하고 어떤 부분을 외주생산할지에 대해 검토 중이다. “지금까지는 공정개발은 내부에서 하고 생산은 외부업체에 이전해서 하였다. 이후로는 어떤 부분은 내부에서 생산하려고 한다. 내부생산은 시간과 품질을 관리하는데 매우 효과적이다. 물론 생산을 하기 위해서는 시설비가 들게 마련이다.”라고 카티레산은 말했다.
기업공개, 초기 임상 및 임상 중단 결정
회사 설립 후 4년간 Verve Therapeutics는 $800M (9,600억원) 이상의 펀딩을 하고 약 $250M (3천억원) 정도를 써서 현재 $550M (6,600억원) 정도가 남아있다. “매출이 나기 이전의 바이오텍 회사는 데이타가 현금이고 우리는 임상시험의 성공가능성을 높일 수 있는 데이타를 만들어서 이 약물의 가능성을 알렸다.” Verve의 현재 현금으로는 2025년 하반기까지 생존할 수 있다고 카티레산은 말했다. 이 기간동안의 중요한 마일스톤은 첫번째 프로그램을 임상에 진입시켜서 VERVE-101이 인간과 유인원에서 유효함을 증명하는 인간 PoC 데이타를 얻는 것이다.
Verve는 2021년 6월에 $300M (3,600억원) 규모의 IPO를 했고 (번역 중입니다)
Verve completed a $300 million IPO in June 2021, and Kathiresan notes that only around 10% of the 2021 IPO class of companies are currently trading above their initial share prices. Verve Therapeutics is currently one of them, but an FDA hold on VERVE-101’s IND application announced in early November sent its stock price tumbling. At press time, no detail about the hold had been released, and Kathiresan declined to provide additional comment on the news. Patients in the U.S. will have to wait for the hold to lift before early-stage human clinical trials can begin.
However, human trials of VERVE-101 are already underway in two countries: New Zealand and the U.K. In July 2022, Verve made headlines with an announcement of the first human ever dosed with a base editing medicine, part of the company’s global Phase 1b trial, called heart-1, which launched in New Zealand. In September 2022, the U.K. Medicines and Healthcare products Regulatory Agency (MHRA) cleared Verve’s application to conduct trials. The FDA’s approval of the company’s IND was expected during the second half of 2022, but more information will be needed from the FDA to know whether or how soon trials can commence stateside.
Speaking prior to the FDA’s IND hold, Kathiresan says the company’s interactions with the FDA have been “very productive,” citing two consultations with the agency: a pre-IND meeting, and before that, an Initial Targeted Engagement for Regulatory Advice on CBER Products (INTERACT) meeting. “We were able to present to them our nonclinical data package, our CMS data package, as well as our clinical protocol, and had very nice feedback from them,” says Kathiresan. In March 2022, the FDA released draft guidance on Human Gene Therapy Products Incorporating Human Genome Editing, which Kathiresan says is “very, very helpful in terms of establishing standards for the field.”
GENE EDITING FOR THE MASSES
Tackling cardiovascular disease with a gene editing therapy cut against the grain in 2018, when Verve began developing its first clinical candidates. While an increasing number of gene editing products in development are now expanding to therapeutic areas beyond rare diseases and cancer, cardiovascular disease remains an ambitious target, due to the size of the potential patient population.
Kathiresan says he thinks about addressable patients in two categories: those with familial hypercholesterolemia, a genetic disease affecting roughly 1 million people in the U.S., and those with garden variety atherosclerotic cardiovascular disease (ASCVD), which affects some 26 million people in the U.S. Men in the former category, on average, have heart attacks in their 40s, and women have heart attacks in their 50s. That means a single-dose gene editing therapy could potentially replace 30 to 40 years of medications and LDL care, says Kathiresan, not to mention the heart attacks, procedures, and other surgeries potentially avoided.
For the latter category, individuals with ASCVD have heart attacks, on average, around age 65. In that group, a single-dose therapy could prevent 15 years of LDL care and other procedures. An ability to successfully treat both groups would provide substantial value, both in terms of cost savings and quality of life. It’s too early in the clinical process to make any decisions about pricing for Verve’s product, but Kathiresan says that when the time comes, the company will “make a case for the value that the medicine will bring to patients, providers, and payers, and then attempt to capture some of that.” However, a dose won’t cost millions of dollars, he says. “That’s not going to be us … we ultimately want to reach millions of patients. It’s not going to be a rare disease pricing model.”
GROWING THE TEAM
What began with a handful of people in 2018 has now grown to 200 employees — no small feat. Kathiresan described his leadership style as “emphasizing speed over perfection,” but in terms of hiring, “we pay a lot of attention to who we bring in, and that’s basically the No. 1 reason for our success.” Once people join the company, they are “nurtured, meaning that we want to understand where they are, where they want to go, and how we can get them there in the context of the company’s goals.” After a comprehensive introduction involving team members and key stakeholders, HR checks in with new employees at 60 days to ensure the new hires are integrating well into the company, that they are getting feedback from their manager, and are clear about career paths. “We provide career ladders which serve as guidelines to help employees understand the different career paths and expectations,” says Kathiresan. Employees are encouraged to create individual development plans (IDPs), and work with managers to align IDPs with personal and company goals. “The IDP serves as a road map for professional development and a tool to prompt deeper development conversations.”
In 2021, Verve launched a leadership development program for VP-level professionals, consisting of a Hogan Assessment, a professional development plan, and several executive coaching sessions. For first-time managers, the company created an emerging leaders program including six development workshops designed to increase engagement, teach effective communication, and help employees become better coaches and managers. For scientific professionals, Verve hosted a seven-session series on creating and delivering powerful presentations to help strengthen design, strategy, and presentation skills. Group-level “insights discovery workshops” are used to “engage employees early in their tenure at Verve by helping employees understand themselves and their colleagues so they can have more respectful and positive working relationships,” adds Kathiresan.
One key recruiting factor stems from the overarching simplicity of Verve’s mission, which is easy for anyone to understand: the eradication of heart attacks. “Everybody knows somebody who has had a heart attack, so it’s a relatable mission.” That may seem obvious, but Kathiresan says choosing a straightforward company mission or goal that solves a clinical problem that patients and physicians care about is more important than it may appear. “People often get lost on whiz-bang technologies and platforms, but at the end of the day, it’s all about making a product that helps patients, ideally one that solves a problem that a lot of people care about.”
Verve has lots of challenges ahead, but Kathiresan is confident that the team he built is ready to solve new problems, many of which don’t have an existing playbook. To do so, he empowers his team to lead, stressing that gut instinct and listening to others are equally as important as data when it comes to making decisions. “Being a successful leader, in a lab and in a biotech company, is rooted in relationships and ‘EQ,’ or emotional intelligence,” says Kathiresan. “Leadership is more about being able to motivate and understand the people around you than it is about pure IQ.”
Sidebar 1
Straight To The Heart
After Kathiresan completed his BA undergraduate degree in history, summa cum laude, at the University of Pennsylvania, he continued his education at Harvard Medical School, beginning in 1992. At that time, cardiology stood out among other fields of medicine in that a variety of useful diagnostic tools existed, and good treatment options were available. From pills to catheter-based procedures and surgery, physicians could make a diagnosis, and choose from a variety of treatment options for patients. “That’s not always the case in medicine … in other areas, you might be able to make a diagnosis and figure out what’s going on, but you might not have the right treatment to go with it,” says Kathiresan.
Despite existing cardiovascular disease diagnostics and treatments, however, the mystery of why some people suffered heart attacks at relatively young ages remained unsolved. “I had quite a few family members who developed a heart attack at a young age, in their early 40s,” says Kathiresan. Heart attacks among multiple people in the same family points to a genetic cause, but when Kathiresan entered the field, the human genome was just being sequenced. “We really didn’t know exactly what letters in the genome conferred risk,” he says.
Kathiresan describes his career as unfolding in three chapters. The first was completing medical school and learning to care for patients with heart disease, which lasted 12 years. The second chapter, also lasting around 12 years, involved learning to conduct research, and leading a lab focused on elucidating the genetics behind heart disease, both in terms of risk and resistance. The third chapter started with the AHA’s One Brave Idea grant competition, which led to the founding of Verve Therapeutics.
The third-chapter goal of creating a “one-and-done” treatment to permanently lower LDL cholesterol levels is based on the knowledge Kathiresan gained during the second chapter of his career. “The main lesson we learned from my 12 years of research into the human genetics of heart attacks is that for individuals with lifelong, consistently low LDL levels, it is really hard to get a heart attack,” he says. “The people with naturally low LDL levels have a DNA mutation that naturally turns off a gene in the liver, a gene that would otherwise raise LDL cholesterol levels. Our idea was to develop a medicine that would mimic the natural situation and turn off that gene.”
