
안녕하세요 보스턴 임박사입니다.
방사선치료는 항암치료의 중요한 부분이지만 비선택적인 문제로 독성 부분이 문제가 되어왔습니다 하지만 최근에 개발된 Radiopharmaceuticals는 특정 암세포 표면항원에 표적함으로써 Precision Radiopharmaceuticals이라는 새로운 분야가 열리고 있습니다. 이를 선도하는 치료제로서 Novartis가 FDA 승인을 받은 Pluvicto (177Lu-PSMA-617) 의 개발 스토리에 대해 글을 적어보려고 합니다.
Pluvicto는 2016년에 독일 University of Heidelberg 의 Clemens Kratochwil 교수팀에 의해 Journal of Nuclear Medicine에 처음으로 보고했습니다. (Picture: Clemens Kratochwil at University of Heidelberg)

2017년 10월에 Endocyte는 ABX GmbH로 부터 Pluvicto (177Lu-PSMA-617)에 대한 exclusive right을 인수하게 됩니다.
Endocyte, Inc. (NASDAQ Global Market:ECYT), a biopharmaceutical company developing targeted therapeutics for personalized cancer treatment, today announced the completion of an exclusive worldwide license of PSMA-617 from ABX GmbH. Endocyte intends to move quickly into Phase 3 development of 177Lu-PSMA-617, a radioligand therapeutic (RLT) that targets the prostate-specific membrane antigen (PSMA), present in approximately 80% of patients with metastatic castration-resistant prostate cancer (mCRPC).
PSMA-617 was developed at DKFZ (German Cancer Research Center) and University Hospital Heidelberg and exclusively licensed to ABX GmbH in Germany for early clinical development.
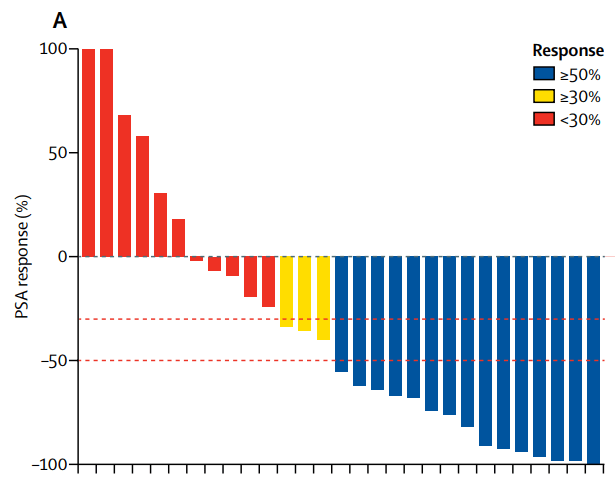
Pluvicto의 임상2상 결과는 2018년 The Lancet Oncology에 발표되었습니다.
그리고 Novartis는 Endocyte를 $2.1 Billion에 인수하게 됩니다.
Novartis has agreed to acquire Endocyte for $2.1 billion, the companies said today, in a deal that expands the buyer’s radioligand therapy (RLT) pipeline of targeted oncology treatments with a Phase III candidate and several early-stage candidates.
Endocyte’s lead candidate is 177Lu-PSMA-617, a potential first-in-class RLT candidate designed to treat metastatic castration-resistant prostate cancer (mCRPC).
Also in Endocyte’s pipeline are additional RLT candidates that include 225Ac-PSMA-617, now in preclinical studies for the treatment of mCRPC. The company has also applied its SMDC platform to develop chimeric antigen receptor T-cell (CAR-T) adaptor molecules, or CAMs, that are each constructed with one FITC molecule combined with a ligand capable of binding to cancer cells.
Endocyte’s planned acquisition comes a year after it agreed to license the RLT candidate from ABX for up to $172 million-plus, and refocus its development efforts around the prostate cancer treatment.
Four months earlier in June 2017, Sherman oversaw a restructuring in which Endocyte eliminated approximately 40% of its workforce, approximately 30 jobs, leaving it with 47 employees. Endocyte further shrunk its workforce last year, ending 2017 with 44 full-time employees, 33 of whom were engaged in R&D activities, according to the company’s Form 10-K annual report, filed February 27.
Endocyte의 Purdue University spin off로 창업부터 Novartis M&A까지 성공스토리는 Purdue University에서 자세히 다루었습니다. Endocyte는 Purdue University Philip Low 교수의 연구결과를 상용화하기 위한 목적으로 설립되었고 오랜기간 공동연구를 해 왔습니다. Philip Low 교수는 Umoja Biopharma의 Co-founder이기도 합니다.
BIOTECH (60) – Umoja Biopharma’s in vivo CAR-T Platform

Founded in the Purdue Research Park, the biopharmaceutical company licensed its first technology through the Purdue Office of Technology Commercialization. Endocyte now has licensed several technologies developed at Purdue, most based on research led by Philip Low, the Purdue University Presidential Scholar in Drug Discovery and the Ralph C. Corley Distinguished Professor of Chemistry.
A turning point for Endocyte came in the fall of 2017 when the company obtained exclusive worldwide rights from a Germany company to develop and commercialize Lu-PSMA-617, an injectable liquid that targets diseased cells with a beta-emitting radioactive isotope, while bypassing healthy cells. A video about Endocyte’s Lu-PSMA-617 can be viewed here.
“We’re working with Dr. Mike Jensen of the Seattle Children’s Research Institute to advance this CAR-T cell therapy into the clinic for the treatment of osteosarcoma, which is typically a pediatric bone cancer,” Low said. “The CAR-T cell program, like our radioligand therapy program is tumor targeted, and so we believe it could be useful for other types of cancers.”
2018년 8월에 발표한 Endocyte Inc의 Corporate Presentation은 아래에 올립니다. 인수당시 파이프라인은 아래와 같습니다.

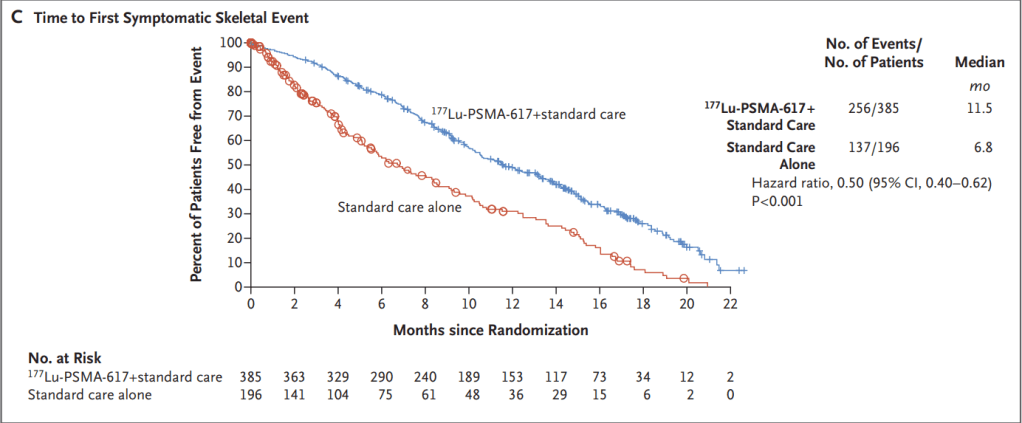
Phase 3 결과는 2021년 New England Journal of Medicine에 보고했습니다.


Novartis has won Food and Drug Administration approval to sell a radiopharmaceutical designed to treat a form of advanced prostate cancer in a key step forward for an area of research the Swiss drugmaker has prioritized in recent years. The FDA cleared the infusion, known as Pluvicto, based on study results showing it could cut the risk of death and slow tumor growth in some of the sickest patients. U.S. regulators also approved Novartis’s diagnostic imaging agent, Locametz, which is designed to help doctors find patients eligible for treatment with Pluvicto, Novartis said in a statement. Locametz is used in a PET scan to identify men whose cancer expresses a certain biomarker.
Novartis delays FDA filing for in-demand radiopharma drug – Biopharmadive 10/24/2023
Novartis is delaying a regulatory submission for its radiopharmaceutical drug Pluvicto in early prostate cancer following mixed results from a late-stage trial, the company said Tuesday as it reported its third quarter earnings. The Swiss drugmaker now plans in 2024 to ask for Food and Drug Administration approval, rather than by the end of this year.
Data released Monday at a medical meeting suggested trial enrollees who were assigned to take Pluvicto at the beginning of the trial might not be living longer than those who received hormone therapy. But the results were affected by patients in the hormone therapy arm who “crossed over” to receive treatment with Pluvicto when their disease progressed.
Novartis recorded $256 million in Pluvicto sales in the third quarter and $707 million for the first nine months of the year, making it the company’s fastest-growing drug. That growth has been aided by “unconstrained” supply after the FDA’s clearance of manufacturing at a factory in Milburn, New Jersey, which could soon be joined by an Indianapolis site now under FDA review.
Pluvicto는 Supply Chain Issue를 해결하기 위한 많은 노력이 있습니다.
Novartis has been compensating for a temporary shortage of Pluvicto with aggressive expansion of manufacturing capacity. The new plant, located in Indianapolis, has won FDA approval to churn out commercial doses of Pluvicto,
Novartis’ radiotherapy production network also includes a Millburn, New Jersey, plant that the FDA cleared to produce Pluvicto for commercial use in April 2023. A site in Ivrea, Italy, has also been supplying the prostate cancer treatment to patients inside and outside the U.S., while a facility in Spain handles ex-U.S. demand.
Further expansions are on the way. Novartis recently unveiled an $85 million plan to build a new radiotherapy facility in China to potentially supply doses for the country starting in 2026. In November, Novartis’ Japan unit said it will invest $100 million in a factory in Sasayama to support radioligand therapy production.
First approved by the FDA in March 2022, Pluvicto quickly became a much-needed option for heavily pretreated patients with PSMA-positive metastatic castration-resistant prostate cancer. Demand exceeded Novartis’ expectations, leading to months of shortages and a halt of new patient starts in early 2023.
Novartis has figured Pluvicto could eventually reach more than $3 billion in peak sales if its late-stage development pans out.
Pluvicto의 Approval Story는 2022년에 Susan Keam에 의해 정리된 적이 있습니다.
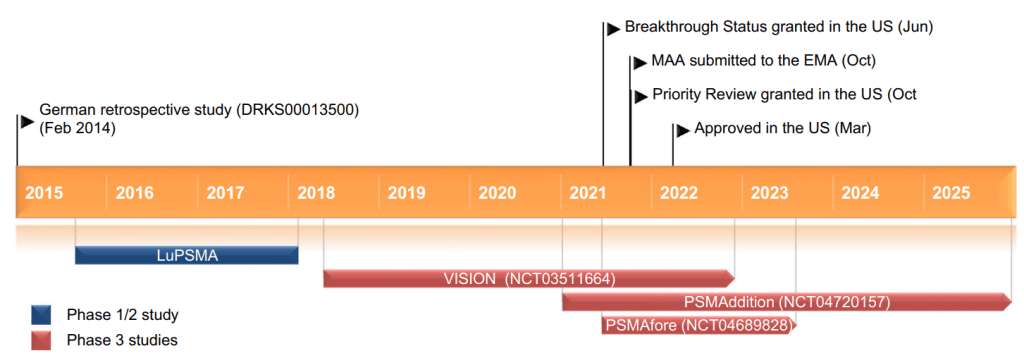
Pluvicto 승인 이후에도 PSMA-targeted Precision Radiopharmaceuticals는 계속 개발 중입니다 이에 대한 임상현황은 아래에 잘 정리되어 있습니다.
