
<보스턴 임박사의 117번째 리포트>
Brown University의 생물공학부 학부생이었던 Josh Cohen과 Justin Klee는 연구논문을 뒤지다가 Neuronal Cell Death에 대해 공부를 하던 중 Phenylbuturate (PB)와 Tauroursodeoxycholic acid (TUDCA)가 각각 효과를 가지고 있다는 것을 발견한다. 아래의 두가지 논문이 대표적으로 이 각각의 약물의 효과를 증명하였다.
그들은 PB-TUDCA combo를 통해서 Neuronal cell death를 막을 수 있다면 Neurodegenerative disease를 치료할 수 있지 않을까? 하는 단순한 아이디어를 가지고 Preclinical test를 하여 PB-TUDCA Combo Hypothesis를 증명한다. 아래 논문이 그 결과를 보여준다.
Amylyx Pharmaceuticals를 설립한다. 경험이 없는 이들에게 Angus Kingon1 교수가 특허출원을 조언하고 Jonathan M. Nelson의 멘토링을 통해 Startup grant와 ALS grant를 확보하고 Preclinical과 임상시험 시작을 할 수 있게 되고 $5 Million Series A을 통해 임상2상을 시작할 수 있게 된다. 임상 2상 CENTAIR 결과를 통해서 FDA 승인을 얻고 판매를 시작했으나 최근 임상3상 데이타가 부정적으로 나오면서 ALS 치료제로 지속할 수 없는 위기에 처하게 되었다. 하지만 아직도 희귀질환 뇌질환 두가지에 대한 임상3상이 계속 진행할 예정이므로 AMX0035가 어려움을 겪고 있지만 약 자체가 실패했다고 볼 수는 없을 것 같다. 기대를 가지고 지켜봐야할 것 같다.
Biomedical engineering alumnus Josh Cohen ’14 partnered with Justin Klee ’13 eight years ago to build a company dedicated to the development of therapeutics for the treatment of neurodegenerative disorders.
In the Cambridge, Mass. conference room where the results of the CENTAUR clinical trial for AMX0035 were delivered to co-founders Josh Cohen and Justin Klee, the excitement was palpable. The study showed that Amylyx Pharmaceuticals, Inc.’s amyotrophic lateral sclerosis (ALS) pipeline drug showed potential to prolong patient survival and slow rapid disease progression in ALS patients, analyzing 137 patients enrolled at 25 top ALS medical centers across the United States.
The functional and survival benefits of AMX0035 significantly strengthened the drug’s position to enter the ALS market over the coming years as a potential disease-modifying therapy. “But the point where it really hit us was when we talked to a nurse at Mass General Hospital in the days following,” Cohen said. “The nurse said, ‘Every day I have to tell people that their life is being cut substantially short. There are a lot of tears in clinic. But today I was able to share a ton of hope. It was a different day.’
“Things like that are why you do it. The patients are why we get out of bed in the morning.” Cohen said, explaining how the patients who participate in Amylyx trials are the motivation behind the company’s progress. “There was a time we were having a significant issue with the manufacturing process, and there just seemed no way to do it. It was a pretty big hold up, and we were in a stage where we had investors, had built up hope, and were not sure of a path forward. After a two-hour meeting with a patient, who was at a stage where he was speaking through an eye tracker but still found joy and laughter, we walked out with a different perspective of what people with ALS were dealing with on a daily basis. We found a way through the issue.”
Klee and Cohen were recent guests on the Brown University Carney Institute for Brain Science’s “Carney Conversations,” where Klee recounted the genesis of Amylyx. “We had been friends since Josh’s first year, and one day he shows up after I hadn’t seen him in long time, and said, ‘I’ve been reading all these papers and thinking about Alzheimer’s and neurodegeneration and I think I may have a way of looking at it and treating it.’”
Then a junior, Cohen was in the literature-heavy portion of his studies. “I just got really into that,” Cohen said. “This idea that there are literally millions of papers out there that are waiting to be read, and integrated – I ended up reading a lot, particularly about neuronal death, trying to look at it in a way that there might be more to try.
“The literature search was really my own, but courses that were making us read a lot at that time were a stem cell engineering course, and Biomaterials with Tayhas Palmore. I took Neuroengineering with Leigh Hochberg, and then my pharmaceuticals interest came from Drug and Gene Delivery with Edith Mathiowitz, and also a Physiological Pharmacology course. Once we started Amylyx, it became my capstone with Dr. (Anubhav) Tripathi.”
Klee was intrigued with Cohen’s early theories, and scientific curiosity led the way for starting the company. “These are such enormous problems,” said Klee. “What if we found a way to treat them that people haven’t yet thought of? This is such an amazing industry. It’s scientifically interesting from both a business and people perspective. And at the end of day, if we’re successful, we’ve helped people who really need it,” he said. “So we kept reading and the short story is, we said ‘Let’s start a company.’ In retrospect, we had no idea what that meant.”
Cohen said Professor Angus Kingon was the engineering adviser the pair leaned on most heavily, and was one of the first people to encourage and offer advice on securing a patent. Alan Harlam, founding director of the Social Innovation Initiative (SII) at Brown’s Swearer Center, and now mentor in the Jonathan M. Nelson Center for Entrepreneurship, was another early adviser of Amylyx. A startup grant from Swearer helped Cohen and Klee scrap together enough money to run the first experiments at Charles River Labs.
“It may look from the outside that Justin and I drove everything, but the truth is every step of the way so many advisers and different people were giving us tips and tricks on how to get to the next stage. Brown is such a great place to be for it with its entrepreneurial spirit, starting with the open curriculum, encouraging self-directed thinking,” said Cohen.
“We had the advantage of not knowing, not having a full appreciation of what it might take to do this. Once we had fallen in love with the idea, we thought if we didn’t pursue it, it wouldn’t be pursued. It wasn’t easy: Inexperienced leaders going after diseases that had historically been impossible, with a project that wasn’t going to be cheap. There were many times we wondered if there was a path forward. A few advisers took a chance on us, and told us to keep pushing. That helped a lot.
“The nice thing about doing this at the undergrad age is if the thing totally fails, it’s not as bad as it could be. If I could boil the last eight years into a few sentences, it would include preparing for unexpected challenges. As we were developing, the hardest thing was manufacturing. The standards that are expected for pharmaceutical manufacturing is a tightrope. And it requires a lot of iteration and a lot of frustration,” Cohen said.
“One summary of the whole process is get a little data, raise a little money, get a little more data, raise a little more money. It makes the whole process very stressful and difficult, because you end up fundraising quite frequently and it’s never far away that you’ll have to be fundraising again,” he said. “One thing that was really transformative for us, one uphill battle we fought, was our credibility, because we are young founders. We had to overcome that, and one of the biggest helps for that was a grant from the ALS Association that helped support the clinical trial. Both the money and implied credibility of that was helpful.
“I’ve always felt Brown doesn’t get enough credit on the entrepreneurship side. The school almost naturally recruits entrepreneurs. The open curriculum means you can take whatever you want, so Brown students take that in all sorts of directions. I mean, there are some whose schedule looks totally indistinguishable from if they went to another school, and there are students who totally focus in one area or take one of everything.
“It naturally selects kids who want to be self-directed, self-motivated, who want to build their own thing, brand, skillset and persona. It’s really exciting what Brown is doing with the Nelson Center to bring that out and operationalize that. One of the things I really appreciate about Brown engineering is the mentality of self-direction. Many of the early classes, and certainly in the later classes, professors are likely to give you a couple of tools and encourage you to figure it out. That can be frustrating, but I loved it. I thought it made you re-think how you approach problems. I still find everything I learned from Brown Engineering incredibly helpful at Amylyx, but especially the problem solving. It’s a hard program, so when you’re done with it, you sort of know how to manage your life, your schedule, and your time. We’ve had a couple of candidates come to interview who are Brown engineers and I always say, ‘Let’s put them at the top of the list.’”
The co-CEO business model, considered by many an unusual way to structure a company, seems to work advantageously for Klee and Cohen. “It helps a lot that we are very good friends,” Klee said. “We have a fundamental trust that we both have the same vision, but we usually approach problems completely differently, me from neuroscience and Josh from biomedical engineering. But I know when Josh comes forward, it is going to be well thought out and something I haven’t thought about. We recognize our strengths and weaknesses and how we each have our different way of doing things. Most big problems you want to talk it through and have a debate, anyway. On the smaller issues, we have decision makers at different places at the same time.”
Cohen added, “Collaboration is everything. If your partners and advisers are unpleasant to work with you won’t get far. Two-way respect is what makes it work. And a lot comes down to self-awareness. None of us is perfect. We realized early what we’re good at, and what we’re bad at.”
With the most recent trial results showing such promise, Amylyx now looks to the future. “The CENTAUR trial showed that people with ALS could retain their functional abilities for more time and survive longer. With that data the question is, is that enough for approval and marketing, or are additional studies needed? We’ve decided to file for approval in Canada and Europe. In the United States, it seems likely we will need additional data. So we’re planning an additional study to support that,” Cohen said. “Our goal now for the company is marketing the drug, building operations and the infrastructure to support that while working to complete any necessary additional trials. We are focused on that right now. We really want to do an excellent launch on this drug candidate for ALS before we look too far past that,” he said.
“We are thrilled about AMX 0035. These are terribly hard diseases, so a significant outcome is awesome, but it is not a cure. As our company continues to grow, we feel we have a social contract to reinvest those resources into driving more and better solutions for neurodegenerative disease. ALS is obviously our focus, but we also have a trial ongoing in Alzheimer’s, and are researching other neurodegenerative diseases as well. This is definitely the space we want to stay in, where we’ve developed our relationships, and have our expertise.”
Amylyx Pharmaceuticals Inc. announced today that it has completed a $5 million Series A financing to support its upcoming Phase II trial in patients with Amyotrophic Lateral Sclerosis (ALS). Morningside Venture led the financing and was joined by the ALS Investment Fund and former Genzyme CEO Henri Termeer, as well as new and previous private investors.
The financing adds to a recently awarded $2.96 million grant to support the clinical trial from the ALS Accelerated Therapeutics Initiative, which is a collaboration between the ALS Association and ALS Finding a Cure. Overall, Amylyx has raised $10 million in grant funding and private financing to advance AMX0035. The IND for this Phase II trial is on schedule for the fourth quarter of 2016 and the trial will start shortly thereafter. Over 20 clinical sites across the United States have already expressed interest in participating.
In connection with the financing, Dr. Isaac Cheng, M.D. from Morningside and Mr. Felix von Coerper from the ALS Investment Fund have joined Amylyx’s board of directors. Mr. Termeer will become a Board observer. They will join directors Dr. George Milne, former President of Central Research and President of Strategic and Operations Management at Pfizer; Dr. Walter Gilbert, Nobel Laureate and former Biogen CEO; and Stephen D. Chubb, previous CEO of three publicly-traded biotechnology companies and previous chairman of the board at Mount Auburn Hospital.
“Amylyx has built a strong rationale for AMX0035 in the treatment of ALS and has developed an outstanding clinical plan to evaluate its potential in ALS patients,” said Dr. Isaac Cheng M.D. of Morningside. “There is a critical unmet need for ALS treatments and Morningside is pleased to support Amylyx’s program.”
“With AMX0035, Amylyx seeks to advance a promising approach to ALS therapy, by targeting neuroinflammation and cell death, that is now poised to enter human studies,” said Mr. von Coerper. “Our investment in Amylyx is aligned with the Fund’s goals of driving medical advancement for this terrible disease.”
“Amylyx’s clinical plan is not only designed to validate their therapeutic candidate, but also push forward important innovation in trial design and execution in ALS,” said Henri Termeer. “I am excited to work with Amylyx as they work to make a difference in the lives of these patients.”
AMX0035 combines two drugs, sodium phenylbutyrate (PB) and tauroursodeoxycholic acid (TUDCA), that Amylyx has shown act synergistically in preclinical models of ALS. Previous studies with PB and TUDCA as single agents demonstrated efficacy in several cellular and animal models of ALS. In addition, PB and TUDCA have been individually tested in clinical trials of ALS and both showed safety and tolerability and preliminary signs of efficacy.
The Phase II clinical trial will test the safety and tolerability of AMX0035, as well as functional outcomes. Analysis of biomarkers of cell function, neuronal damage, and inflammation will be included as a major part of the trial, along with a new measure of muscle strength that has the potential to be an objective and sensitive assessment of disease progression.
“We are very pleased with the support and commitment of this group of highly experienced investors who share the focus of making a significant difference in this disease,” said Justin Klee, President at Amylyx. Josh Cohen, CEO added, “We are also thrilled to welcome our new Board members, and anticipate benefiting from their guidance as we prepare to enter clinical development with AMX0035.”
ALS is a progressive neurodegenerative disease that affects nerve cells in the brain and the spinal cord. Eventually, people with ALS lose the ability to initiate and control muscle movement, which leads to total paralysis and death, usually within two to five years of diagnosis. There is no cure, and only one drug approved by the U.S. Food and Drug Administration modestly extends survival.
Amylyx raises $135M to gear up for potential Canadian launch of ALS drug – Fierce Biotech 7/20/2021
A month after filing for Canadian approval for its lead ALS candidate, Amylyx Pharmaceuticals has picked up $135 million to bankroll a potential commercial launch and support a pipeline of other candidates for neurodegenerative diseases, including the one causing a stir this summer: Alzheimer’s disease.
The Cambridge, Massachusetts-based biotech raked in the oversubscribed series C Tuesday to support the clinical development and launch plans, pending a Canadian green light for the amyotrophic lateral sclerosis (ALS) treatment. The novel combination of compounds called AMX0035 was found to slow the progression of ALS in a phase 2/3 study presented last September.
Viking Global Investors led Tuesday’s financing round, with participation from Bain Capital Life Sciences, Perceptive Advisors and about a dozen other backers.
AMX0035 combines two orphan drugs—taurursodiol and Buphenyl—into an oral medication that works to protect brain cells’ energy-producing mitochondria and bolster the endoplasmic reticulum, ensuring constructed proteins fold and operate correctly.
The therapy was put up for a Health Canada nod last month and Amylyx intends to submit for a marketing approval with the European Medicines Agency by the end of this year. FDA regulatory updates are to come, the company said.
Amylyx also presented a trial design for an international phase 3 study of the drug in May.
The biotech has beefed up its board and leadership in recent months to support a commercial roll-out, including the additions of Amicus Therapeutics CFO Daphne Quimi and Paul Fonteyne, the former president and CEO of Boehringer Ingelheim’s U.S. operations. Amylyx also hired Chris Aiello in April to lead operations in Canada as general manager, after leading the rare disease and rare blood disorder units at Sanofi Genzyme’s Canadian outfit.
Amylyx also landed a new CFO in January with a direct connection to the ALS movement, picking up former Alkermes CFO James Frates, who is the cousin of the driving force behind the Ice Bucket Challenge. Funds raised from the viral ALS awareness social media movement helped support Amylyx’s own trial.
The 2020 Fierce 15 winner raised $30 million last summer for AMX0035 and a midstage trial for a potential Alzheimer’s treatment. Last November, Amylyx said the last patient had completed the phase 2 trial, dubbed Pegasus, with top-line data due in the first half of this year. The company’s website now says the read out will happen sometime this year.
The company could benefit from the revived interest in Alzheimer’s following the FDA’s controversial approval of Biogen’s Aduhelm. The decision has been criticized by federal, congressional, industry and hospital system stakeholders alike, but competing companies have seen a rise in investment interest.
Amylyx Pharma’s IPO lands $190M as ALS drug begins FDA review – Med City News 1/7/2022
Amylyx Pharmaceuticals has joined the public markets with a $190 million IPO that will support the company as it shepherds its amyotrophic lateral sclerosis drug through regulatory review, and if all goes well, a commercial launch as a new treatment for the neuromuscular disorder.
Cambridge, Massachusetts-based Amylyx boosted the size of the offering by 1.25 million shares. Initially planning to offer 8.75 million shares, Amylyx ended up offering 10 million shares at $19 apiece, which was the midpoint of the targeted $18 to $20 range. Those shares began trading on the Nasdaq Friday under the stock symbol “AMLX.” There was no pop for Amylyx’s stock price, which closed its first day of trading at $18.07.
Amylyx aims to treat ALS by addressing the death of neurons. The company’s drug, AMX0035, is comprised of two small molecules that each take a different approach to pathways associated with neuronal survival. The company contends that the two mechanisms combined offer the potential to help neurons live longer. In a placebo-controlled Phase 2 study, patients treated with the Amylyx drug showed improvement on measures of physical function in ALS patients. Based on those results, Amylyx submitted a new drug application to the FDA. Last week, the agency accepted that application under priority review and set a June 29 target date for a regulatory decision.
Before the FDA makes a decision, it will convene an independent panel of experts to discuss AMX0035’s safety and efficacy as well as any scientific questions regarding the drug. The date for that advisory committee meeting has not yet been set. In the meantime, a larger Phase 3 study is underway. The FDA went back and forth on whether the larger study was needed to support a drug application. Though the agency ultimately concluded that Amylyx could seek approval based on the Phase 2 data, the company is proceeding with the late-stage study to generate additional data on the drug’s safety and efficacy as it pursues regulatory approvals in other markets around the world.
According to the IPO filing, Amylyx traces its beginnings to a Brown University dorm room where, in 2013, co-founders Josh Cohen and Justin Klee set out to find an answer to the question of why neurons die. The duo’s research led to the development of AMX0035, the company’s only drug candidate so far. Though ALS is the drug’s first disease target, Amylyx notes that improving neuron survival has potential applications in many neurodegenerative disorders. A clinical trial is already underway testing AMX0035 in Alzheimer’s disease and tests in Wolfram disease are also planned.
Since its formation, Amylyx has raised about $234 million, most recently a $135 million Series C round of funding last July. Morningside Venture Investments is Amylyx’s largest stockholder, owning an 18.9% post-IPO stake, according to the prospectus. ALS Invest 1 owns 10.8%, while Viking Global Investors owns 8.8%.
As of the end of the third quarter of 2021, Amylyx reported a $125.7 million cash position. Combined with the IPO proceeds, the company plans to deploy about $100 million of its capital toward the regulatory review process of AMX0035 in ALS, as well as preparation for the potential launch of the drug—if it’s approved. Another $15 million is set aside to fund the ongoing Phase 3 clinical trial through to completion; $10 million is earmarked for expanding the company’s drug pipeline to other neurodegenerative disorders.
After a long road filled with scrutiny and uncertainty, Amylyx’s amyotrophic lateral sclerosis (ALS) drug, known as AMX0035, has finally scored FDA approval.
The drug, now branded as Relyvrio, won approval for the treatment of ALS in adults. It’s the first ALS treatment that showed a significant slowing in both disease progression and functional decline, as well as extended survival, in a randomized clinical trial.
After a first FDA advisory committee meeting in March ended with a negative 6-4 vote for the drug, many companies would have stopped there. But Amylyx was persistent, and the committee reconvened this September to discuss a new analysis the company submitted, resulting in a positive 7-2 vote. The long stretch of time helped the company prepare for an eventual launch, despite not knowing when, or if, its drug would get approval.
“We’ve been planning this a long time,” said Josh Cohen, Amylyx’s co-CEO and co-founder, referring to the drug’s original FDA target decision date of June 29. “We’re fully prepared,” Cohen said in an interview with Fierce Pharma ahead of the approval.
The drug will carry a list price of about $158,000 per year in the U.S., the company said on a post-approval conference call, noting that the price is below the latest FDA-approved ALS product. However, Amylyx is “committed to providing financial assistance” for patients with commercial insurance and will provide the drug at no cost for underinsured or uninsured patients who meet “certain financial eligibility criteria” and who have “exhausted all other options,” said Amylyx’s chief commercial officer Margret Olinger.
The price, before being revealed, was already criticized by U.S. watchdog ICER in June. Using a placeholder price of $169,000 per year, the organization said the cost-effectiveness of the medicine would “far exceed typical thresholds.”
The FDA based its approval on data from the phase 2 Centaur trial and biomarker results from a phase 2 of the drug in Alzheimer’s disease. At the recent FDA expert panel meeting, some panelists voiced a desire to wait for the results of the phase 3 Phoenix trial. The company doesn’t expect top-line results from that trial until 2024, Justin Klee, Cohen’s counterpart as co-CEO and co-founder, said in an interview with Fierce Pharma.
“For people with ALS, that’s a long, long time,” Klee said. If the agency were to wait until the results of that trial came out, “a whole generation of ALS patients will die without access,” Cohen added. “It seems against common sense to deny that to people.”
From pricing to insurance to supply, Amylyx is set and covered, Cohen said. But the company wants to do more than “just launch a drug into the market.”
“ALS has a lot of other things that need changing,” Cohen said. From diagnoses that take too long, “woefully underfunded” clinics, and challenges barring access for patients, there are a number of unaddressed, long-standing problems that need addressing, and that Amylyx wants to “put a pretty significant effort” into fixing.
“ALS has a lot of other things that need changing,” Cohen said. From diagnoses that take too long, “woefully underfunded” clinics, and challenges barring access for patients, there are a number of unaddressed, long-standing problems that need addressing, and that Amylyx wants to “put a pretty significant effort” into fixing.
Amylyx Pharmaceuticals, Inc. (NASDAQ: AMLX) (“Amylyx” or the “Company”) today announced topline results from PHOENIX, a global, 48-week, randomized, placebo-controlled Phase 3 clinical trial of AMX0035 (sodium phenylbutyrate and taurursodiol [also known as ursodoxicoltaurine]; RELYVRIO® in the U.S., ALBRIOZA™ in Canada) in people living with amyotrophic lateral sclerosis (ALS). PHOENIX did not meet its primary endpoint of reaching statistical significance (p=0.667) as measured by change from baseline in the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) total score at Week 48, nor was there statistical significance seen in secondary endpoints. Amylyx plans to present the data from PHOENIX at an upcoming medical meeting and will publish the results in a medical journal later this year.
Amylyx will continue to engage with regulatory authorities and the broader ALS community, including ALS specialists and other multidisciplinary experts, people living with ALS, and advocates, to discuss the results from PHOENIX within the next eight weeks and make informed decisions. Amylyx intends to share plans for RELYVRIO/ALBRIOZA in ALS, which may include voluntarily withdrawing RELYVRIO/ALBRIOZA from the market. At this time, RELYVRIO/ALBRIOZA and its related patient support program will continue to be available for people living with ALS. Amylyx has voluntarily decided to pause promotion of the medication during this time.
We are surprised and deeply disappointed by the PHOENIX results following the positive data from the CENTAUR trial. Our main priority at the moment is sharing the information with people living with ALS and their treating physicians; this is part of our continued commitment to them and our mission. Over the next eight weeks, our team will continue to engage with regulatory authorities and the ALS community to discuss the results from PHOENIX. We will be led in our decisions by two key principles: doing what is right for people living with ALS, informed by regulatory authorities and the ALS community, and by what the science tells us. On behalf of the entire Amylyx team, we are grateful to the ALS community and for the dedication of trial participants, investigators, and study site teams. With data collected from 664 participants in PHOENIX, we are certain there will be important learnings that will help inform future ALS research. We are steadfast in our commitment to the ALS community and our mission, including with AMX0035 where it has shown potential in neurodegenerative diseases such as Wolfram syndrome and progressive supranuclear palsy, and with AMX0114, our investigational antisense oligonucleotide targeting calpain-2, in ALS,” said Justin Klee and Joshua Cohen, Co-CEOs of Amylyx.
PHOENIX Study Results:
The Phase 3 PHOENIX study enrolled 664 adults living with ALS. Participants were randomized three-to-two to receive either AMX0035 or placebo, with both treatment groups receiving standard-of-care. Continuation of a stable dosing regimen of riluzole and/or edaravone was permitted.
- PHOENIX did not meet the primary endpoint: There was no significant difference observed between participants treated with AMX0035 and placebo in ALSFRS-R total score change from baseline at Week 48 (p=0.667). No significant difference was observed in the subset of participants who met the CENTAUR trial criteria. There were also no significant differences observed across secondary endpoints.
- Consistent safety and tolerability profile: AMX0035 was well-tolerated in PHOENIX. There were no new safety signals, reinforcing the favorable and manageable safety profile observed with AMX0035 to date.
- European participants who completed the 48-week randomized phase had the option to enroll in an open label extension of the trial of up to two years in duration, which remains ongoing.
Science of AMX0035:
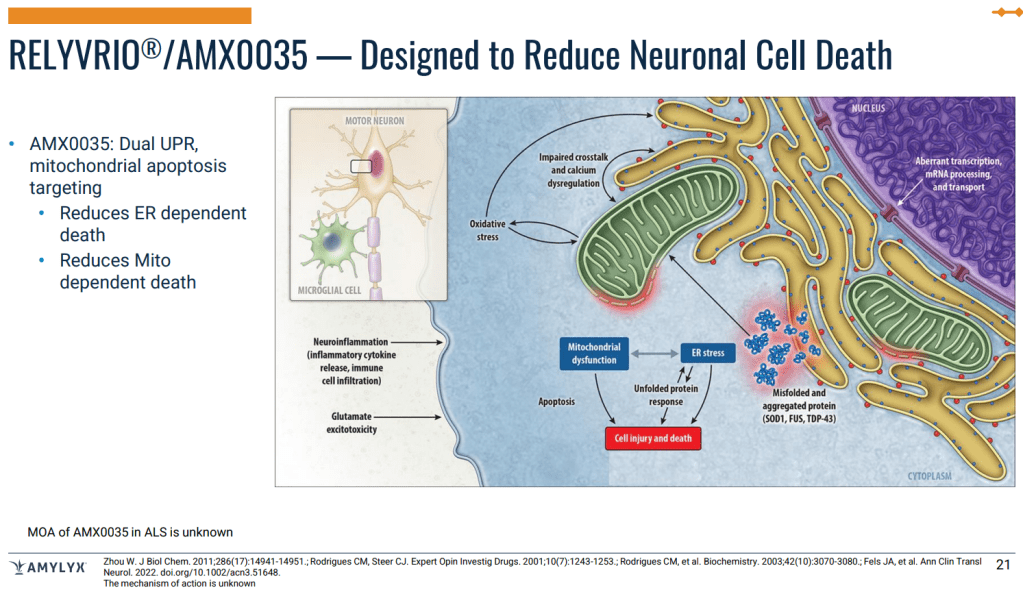
AMX0035, a specially formulated oral fixed-dose combination of PB and TURSO, has been shown in numerous preclinical studies to have a robust, synergistic effect in targeting two different destructive neurodegenerative disease pathways by mitigating endoplasmic reticulum stress and the associated unfolded protein response and mitochondrial dysfunction thereby reducing neuronal cell death. Additionally, AMX0035 has been shown to also reduce markers associated with neurodegenerative diseases in clinical trials, including a reduction of tau, a key protein aggregate shared across several neurodegenerative diseases, and YKL-40, a marker of neuroinflammation.
Update on Ongoing AMX0035 Studies:
The global, randomized, double-blind, placebo-controlled Phase 3 ORION clinical study of AMX0035 in PSP remains ongoing. The first participant was dosed in December 2023, and the Company is planning for an interim analysis. Topline results continue to be anticipated in 2025 or 2026.
Data from the ongoing 12-participant, single site, open-label Phase 2 HELIOS clinical study are demonstrating evidence of clinical activity of AMX0035 in Wolfram syndrome. This study is fully recruited, and the Company plans to present preliminary data in the second quarter of 2024.
After trial failure, will Amylyx pull ALS drug Relyvrio off the market? – Fierce Pharma 3/8/2024
After reporting the failure of a confirmatory trial of its amyotrophic lateral sclerosis (ALS) drug Relyvrio (AMX0035), Amylyx Pharmaceuticals is uncertain whether it will pull the treatment from the market in the U.S. and in Canada, where it is known as Albrioza.
“We’ll spend the next eight weeks engaging with regulatory authorities and the ALS community to share the top-line data,” co-CEO Justin Klee said on a Friday conference call. “We’ll follow the science and do what’s right for the community which may include voluntarily removing the product from the market.”
The 11-year-old Cambridge, Massachusetts-based company has decided to pause promotion of Relyvrio. Patient support services will remain in place, it said.
Two weeks ago, Amylyx reported sales of Relyvrio at $380 million in its first full year on the market, following its approval in September 2022. While sales initially scaled up quickly, the trajectory had slowed, going from $103 million in the third quarter to $108 million in the fourth quarter.
Other than to report that the PHOENIX trial did not meet its primary endpoint, failing to reach statistical significance as measured by change from baseline in the revised ALS functional rating scale (ALSFRS-R), or any of its secondary objectives, Amylyx did not reveal figures from the 48-week trial that enrolled 664 ALS patients.
The company said it will present the data at an upcoming medical conference, and results will be published in a medical journal later this year.
With the devastating news, Amylyx’s shares plummeted 83% by midmorning.
“The news comes as a massive disappointment—not only for us (where AMLX has been a top pick) and investors—but also to ALS patients and the overall ALS community,” analysts from Mizuho Securities wrote in a note to clients.
The company said it will continue to study AMX0035 as a treatment for two other neurodegenerative diseases—Wolfram syndrome and progressive supranuclear palsy. Amylyx also hopes to enter the clinic in the second half of this year with AMX0114, a treatment for ALS patients designed to lower their levels of calpain-2 to strengthen their nerve fibers.
After much consternation and two advisory committee meetings—one voting down AMX0035 6-4 and the other giving it a thumbs-up vote of 7-2 after new analysis of trial data—the FDA signed off on Relyvrio 19 months ago.
The decision was based on results of the phase 2 CENTAUR trial, which showed that AMX0035 slowed progression and functional decline and extended survival. Experts who recommended rejection urged the FDA to wait until results of the phase 3 PHOENIX trial were available.
On Friday, Klee would not speculate on the difference between the trial results.
“I think it’s early,” Klee said. “It’s particularly important to take time to meet with the ALS experts. I think it speaks to the heterogeneity of ALS and also the difficulty in ALS and neurodegenerative diseases more in general. It’s so imperative that when we all, fighting these diseases, have setbacks like this, we learn from them so we can continue to advance because I do very strongly believe that we can have meaningful advances for people with neurodegenerative diseases.”
The ALS Association was heavily invested in the success of the drug as well. Through its Ice Bucket Challenge in 2014, the organization donated $2.2 million to assist in its development. It also collected more than 50,000 signatures and submitted them to the FDA in 2020, urging the regulator for approval.
“While today’s news is disappointing, there are more than 50 potential treatments in the clinical stage of development, including more than a dozen in phase 3 trials,” the ALS Association said in a statement. “We are more committed than ever to ensuring that safe and effective treatments are approved and available to people living with ALS as quickly as possible.”
