BIOTECH (74) – Obsidian Therapeutics OBX-115 (on-switch cytoTIL15 Therapy)
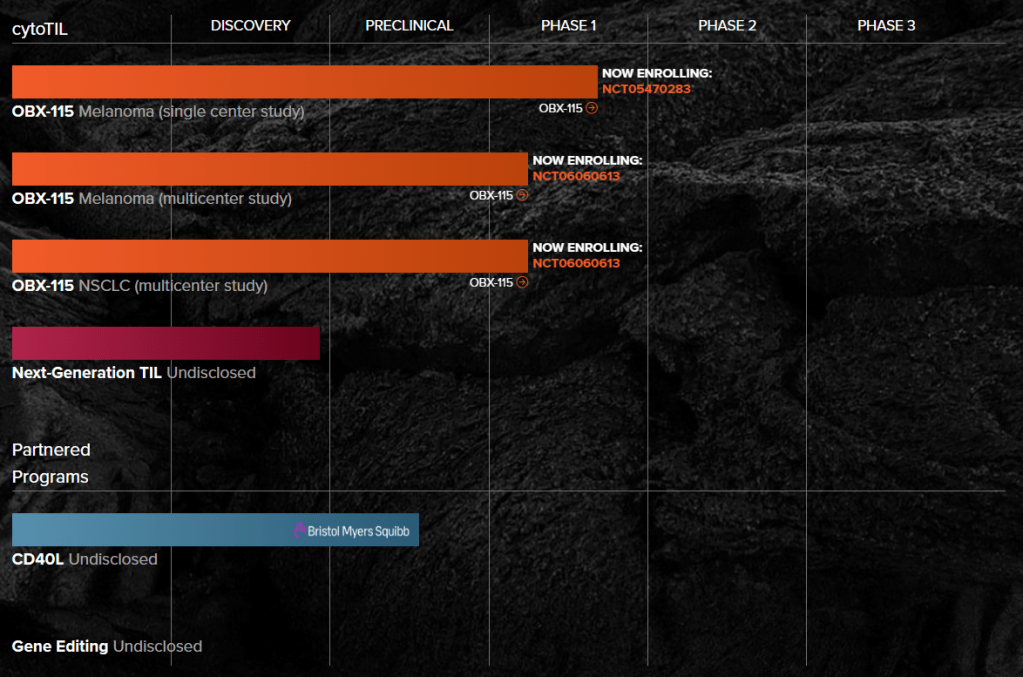
Obsidian Therapeutics, Inc., a clinical-stage biotechnology company pioneering engineered cell and gene therapies, today announced that Bristol Myers Squibb (NYSE:BMY) has opted to extend the term of the parties’ multi-year strategic collaboration for the discovery and development of novel, regulated cell therapies that utilize Obsidian’s cytoDRiVE® technology for the controlled expression of the immune enhancer CD40L. Today’s announcement builds on the existing relationship between Obsidian and Bristol Myers Squibb, initiated in 2019, and follows the first opt-in decision by Bristol Myers Squibb in 2020.
“We are delighted to extend our productive strategic partnership with Bristol Myers Squibb, an industry leader in the field, to advance next-generation cell therapies to patients with solid tumors and other malignancies,” said Paul K. Wotton, Ph.D., Chief Executive Officer of Obsidian Therapeutics. “This announcement comes at an exciting time for Obsidian as our own lead program using cytoDRiVE technology enters the clinic.”
This multi-year collaboration extension provides Bristol Myers Squibb with the exclusive option to in-license worldwide rights for cell therapy candidates incorporating Obsidian’s cytoDRiVE technology to control the expression of CD40L for the treatment of cancer. Under the terms of the agreement, Obsidian is eligible to receive potential future milestone and royalty payments.
Obsidian reports positive data from melanoma cell therapy trial – Clinical Trials Arena 12/13/2023
Obsidian Therapeutics has reported positive top-line data from a Phase I clinical trial of its cell therapy candidate, OBX-115, to treat metastatic melanoma.
The first-in-human trial that is underway is designed to analyse the efficacy and safety of OBX-115 in patients with melanoma that has relapsed and/or is refractory to previous treatment with an immune checkpoint inhibitor (ICI).
An objective response rate (ORR) of 50%, with 33% complete responses, was reported at a median follow-up period of 18 weeks.
Disease control rate in the trial was 100%, with responses intensifying over time.
No dose-limiting toxicities were reported in the trial, and the treatment-emergent adverse event profile was in line with that of lymphodepletion.
A tumour-infiltrating lymphocyte (TIL) cell therapy with pharmacologically regulatable membrane-bound IL15, OBX-115 has the potential to become a therapeutic option for melanoma and other solid tumours.
Obsidian Therapeutics chief development officer Parameswaran Hari said: “The OBX-115 data show its potential to be a meaningful advancement in the treatment of metastatic melanoma and TIL cell therapy.
“These initial topline results support the promise for OBX-115 to drive responses in this heavily pre-treated patient population and facilitate the expansion of TIL cell therapy in melanoma to a broad group of patients, without the need for IL2 [interleukin-2].”
In another development, the company enrolled the first subject in a multicentre Phase I/II clinical trial of OBX-115 in patients with advanced or metastatic melanoma who are resistant to ICI therapy.
Obsidian Therapeutics CEO Madan Jagasia said: “These positive results underscore the potential for OBX-115 TIL cell therapy to offer patients with metastatic melanoma a differentiated TIL therapy, without the need for IL2.
“Furthermore, the emerging profile of OBX-115 indicates it will allow expansion of TIL cell therapy into a broad patient population, including those who may not be able to tolerate IL2 or choose not to receive it.”
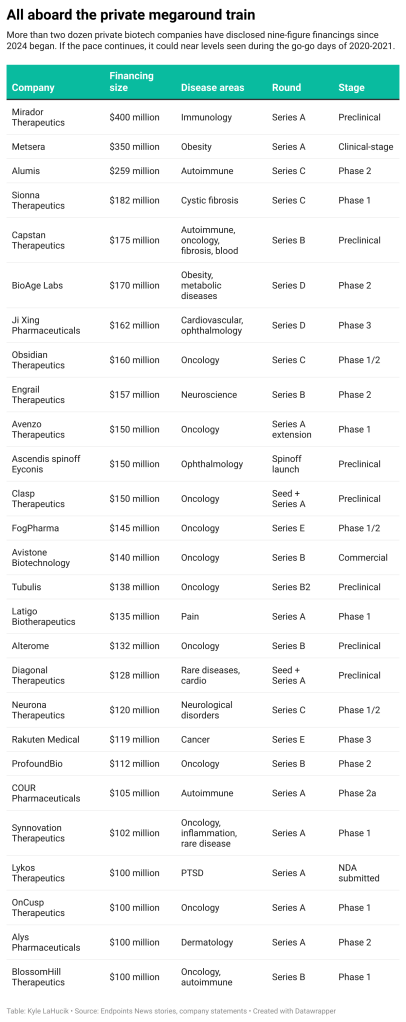
Obsidian Therapeutics rakes in $160M for solid tumor cell therapy program – Endpoints News 4/3/2024
Obsidian Therapeutics announced Wednesday that it raised $160.5 million to push its tumor-infiltrating lymphocyte (TIL) program further in clinical trials.
The Series C raise comes after Obsidian reported initial data from six patients with advanced melanoma who received its tumor-infiltrating lymphocyte cell therapy. With the new $160.5 million, Obsidian plans to push its TIL therapy, OBX-115, forward in multicenter studies in both melanoma and non-small cell lung cancer.
“On the back of that press-released data, we launched into the journey of the Series C financing,” Obsidian CEO Madan Jagasia told Endpoints News in an interview. In December, Obsidian shared that three of six melanoma patients responded to its TIL therapy at a median follow-up of about four months, with two of those patients going into complete remission.
The Series C was led by Wellington Management and included Foresite Capital, Atlas Venture, Bristol Myers Squibb, Novo Holdings, RTW Investments and RA Capital Management, among many others.
In February, Iovance Biotherapeutics won the first FDA approval for a TIL therapy, Amtagvi, also in melanoma. In Amtagvi, the cells are not engineered before they are expanded and are reliant on interleukin-2, which is used both during the manufacturing process and administered to patients after they receive the cell therapy. Obsidian believes that its engineered TIL therapy can do away with that reliance on IL-2.
“You are limiting your patient access because only a subset of patients are fit enough to tolerate high-dose IL-2 in the TIL regimens,” Jagasia said. “The approach that we took is — and it’s been known for a while — IL-15 can replace IL-2 in manufacturing.
“What it allows us to do fundamentally is deliver TIL cell therapy without exposing the patient to a high-dose IL-2 and expand the market opportunity,” Jagasia added. The idea is that Obsidian’s drug can be an outpatient cell therapy treatment.
Obsidian plans to complete its mid-stage studies in 2025, as well as complete key manufacturing milestones required by the FDA with its Series C funding, which would set the stage for registrational studies of OBX-115 down the line.
“Clinical data backed up by good translational science should be able to cut through the noise of the market and then get rewarded,” Jagasia said. “I’m optimistic that if the data remains consistent, and the macro economics remain stable, there would be an opportunity to take this to an IPO at the right time in the clinical development journey.”
Obsidian closed its $115 million Series B raise in September 2021 and a Series A round worth $49.5 million in December 2017.