(Picture: Marcis Souza, CEO of Praxis Precision Medicines)
안녕하세요 보스턴 임박사입니다.
미국 바이오텍 기업들이 나스닥 상장 이전에는 펀딩 이력을 꼭 정확히 공표하지 않는 경우도 있습니다. 회사의 경쟁력을 확보하기 위해서 Stealth Mode로 오랜기간 있는 경우가 있죠.
Praxis Precision Medicines는 Private Equity Firm인 Blackston Life Sciences의 펀딩으로 오랜기간 파이프라인 준비를 하고 2020년이 되어서야 $100 Million 펀딩 소식을 발표했습니다. 이 펀딩은 시리즈 B인 것으로 보입니다. 파이프라인 중 가장 앞선 것은 allosteric GABAA modulator인 PRAX-114였고 Calcium channel blocker인 Ulixacaltamide (PRAX-944)가 뒤를 따르고 있다고 발표했습니다.
Praxis uncloaks with $100M to push precision medicines for brain disorders – Fierce Biotech 5/4/2020
Praxis Precision Medicines is coming out of the shadows with $100 million from the likes of Blackstone Life Sciences and a pipeline of central nervous system (CNS) programs. The cash will bankroll a pivotal study for a depression drug as well as push earlier stage programs into the clinic.
As suggested by its name, Praxis aims to do for brain disorders what precision medicine has done for cancer treatment. Its pipeline targets genes that control the imbalance in neuronal signaling that underlies CNS disorders, both prevalent and rare. Since its inception, the Cambridge, Massachusetts-based biotech has banked $100 million from the likes of Novo Holdings, Vida Ventures and Eventide.
Praxis has two candidates in phase 2: PRAX-114, a positive allosteric modulator of GABAA receptors in development for major depressive disorder and perimenopausal depression, and PRAX-944, which blocks a type of calcium channel to treat essential tremor.
“As was achieved in oncology decades ago, recent genetic insights have presented meaningful opportunities to treat brain disorders in entirely different and targeted ways based on the specific genetically validated pathways driving a patient’s disease,” said Kiran Reddy, M.D., a venture partner at Blackstone Life Sciences, Praxis’ founding investor. “We are reducing these insights to practice, to create novel medicines that could fundamentally alter the treatment path and outcomes for patients with brain disorders.”
Reddy’s fellow founders include Chief Scientific Officer Steven Petrou, Ph.D., director of the Florey Institute of Neuroscience and Mental Health at the University of Melbourne in Australia, and David Goldstein, Ph.D., who leads the Institute for Genomic Medicine at Columbia University.
At the helm is Marcio Souza, who joined from PTC Therapeutics, where he has worn several hats since 2014, most recently serving as its chief operating officer.
Over the next year, Praxis plans to start a pivotal study of PRAX-114 in major depressive disorder and release proof-of-concept data for PRAX-944 in essential tremor. It also aims to push its earlier-stage programs into the clinic, including treatments for rare epilepsies.
Praxis Precision Medicines, a clinical-stage, genetic neuroscience company, launched today with more than $100 million in financing since its inception from premier life science investors led by founding investor Blackstone Life Sciences (via prior Clarus funds in 2016) as well as Novo Holdings, Vida Ventures, Eventide and other prominent funds. Praxis is deploying a precision medicine approach to develop high-impact therapies that target the underlying causal mechanisms of both prevalent and rare brain disorders with overlapping disease biology.
“Neurology and psychiatry are finally primed for a revolution in how new therapies are discovered and developed, and Praxis’ approach has the potential to change the treatment landscape,” said Marcio Souza, president and chief executive officer of Praxis. “The combination of the expertise of our team in CNS drug development, insights into CNS biology, and our approach to drug discovery, positions us as at the forefront of the development of novel CNS therapies.”
“Depression, and more broadly, psychiatric and neurological disorders are a large and increasing unmet medical need with profound implications on the economy. Praxis aims to leverage the recent breakthroughs in genetics to develop innovative medicines that can improve the lives of the many patients who need them,” said Nicholas Galakatos, Ph.D., chairman of the Praxis board of directors and global head of Blackstone Life Sciences.
Praxis Approach and Pipeline
Praxis is leveraging recent discoveries in the genetics of epilepsy, which have elucidated genes, that when dysregulated, drive a range of neuropsychiatric and movement disorders. Using these insights, Praxis is rapidly advancing a pipeline of treatments that specifically address genes controlling the imbalance of excitation and inhibition of neuronal circuitry at the core of multiple CNS disorders. The company’s portfolio is led by PRAX-114, a GABAA positive allosteric modulator (PAM) in Phase 2 development for the treatment of major depressive disorder (MDD) and perimenopausal depression, and PRAX-944, a T-type calcium channel blocker, in Phase 2 development for the treatment of essential tremor. Within the next year, Praxis plans to initiate its first pivotal trial for PRAX-114 in MDD, report proof-of-concept data for PRAX-944 in essential tremor and advance its earlier stage programs into clinical development for rare epilepsies and other neurological disorders with genetically validated mechanisms.
“As was achieved in oncology decades ago, recent genetic insights have presented meaningful opportunities to treat brain disorders in entirely different and targeted ways based on the specific genetically validated pathways driving a patient’s disease,” said Kiran Reddy, M.D., co-founder and member of Praxis’ board of directors. “We are reducing these insights to practice, to create novel medicines that could fundamentally alter the treatment path and outcomes for patients with brain disorders.”
Internationally Recognized Founding Team
Praxis’ founders are renowned scientists and clinicians leading the industry’s growing understanding of the genetics and biology of disease-causing targets in psychiatric and neurologic disorders.
Dr. Reddy is a venture partner and senior advisor at Blackstone Life Sciences. He was previously on the corporate strategy leadership team at Biogen and was an associate partner at Third Rock Ventures, where he co-founded multiple biotech companies. Dr. Reddy is a neurologist and started his career in academic medicine at Harvard/Massachusetts General Hospital.
Co-founder David Goldstein, Ph.D. is the Director of The Institute for Genomic Medicine, and Professor in the Department of Genetics and Development, at Columbia University. Dr. Goldstein’s work focuses on broad aspects of precision medicine and is widely recognized for multiple influential studies in population and human genetics, including those of the Epi4K consortium that discovered and characterized novel epilepsy genes.
Co-founder and chief scientific officer, Steven Petrou, Ph.D. is the Director of the Florey Institute of Neuroscience and Mental Health, Head of the Department of the Florey Institute at the University of Melbourne in Australia, heads the Ion Channels and Human Disease Laboratory. Prof. Petrou is a recognized leader in the field of ion channel neuropathies in rare pediatric epilepsies and other neurodevelopmental disorders. His published interdisciplinary research has focused on functional genetics and genomics of epilepsy, elucidation of mechanisms of disease, development of several of the first animal models of genetic epilepsy and discovery and evaluation of consequent precision medicine approaches.
Successful Biotech CEO and Leadership Team
Prior to joining Praxis as president and chief executive officer, Mr. Souza was at PTC Therapeutics, where he served in leadership roles since 2014, most recently serving as chief operating officer. He also served in leadership roles in the U.S. and globally at NPS Pharmaceuticals, Shire and Genzyme. Mr. Souza holds a degree in pharmacy and biochemistry from University of São Paulo and an MBA from Fundação Dom Cabral in Brazil.
The Praxis leadership team is comprised of recognized leaders in neuroscience drug discovery, development, and commercialization, including Bernard Ravina, M.D., M.S., chief medical officer; Stuart Chaffee, Ph.D., MBA, chief business officer; Dr. Petrou., co-founder and chief scientific officer; Marion Wittmann, Ph.D., vice president of biology; Gabriel Martinez, Ph.D., vice president of chemistry; Rosa Tarng, vice president, portfolio management; and Karl Hansen, Ph.D., senior vice president, CMC.
About Praxis Precision Medicines
Praxis Precision Medicines is a clinical-stage genetic neuroscience company developing breakthrough therapies for patients and families affected by complex and debilitating brain disorders, including psychiatric disorders, movement disorders and rare pediatric epilepsies. These disease areas share overlapping genetics and neurocircuit biology, as well as a profound need for new therapeutic options that target the underlying cause of the disease. Praxis is advancing a pipeline of breakthrough medicines with the potential to more precisely treat brain disorders. For more information, please visit www.praxismedicines.com.
시리즈 B를 발표한지 2달만에 시리즈 C-1 $110 Million을 발표했습니다. 앞의 두개 파이프라인에 간질 및 통증 치료제인 PRAX-562가 추가된 것으로 공개했습니다.
Praxis raises $110M to advance 3 clinical-phase CNS drugs – Fierce Biotech 7/28/2020
Praxis Precision Medicines has raised $110 million to take its three clinical-phase central nervous system treatments forward. The pipeline is led by a GABAA positive allosteric modulator that is closing in on the start of a pivotal trial in depression.
Praxis kept a low profile in its early years, only breaking cover to disclose financings in Securities and Exchange Commission filings and publish the starts of clinical trials on registry platforms. The secretive approach was made possible by the support of deep-pocketed founding backer Blackstone Life Sciences, nee Clarus.
By the time Praxis uncloaked in May, the biotech had raised more than $100 million and moved to within one year of the start of a pivotal trial of its lead asset. That candidate, PRAX-114, was trailed closely by another phase 2 asset, PRAX-944, and a then-undisclosed prospect that has now joined the two more advanced prospects in the clinic.
With three clinical programs underway and a pivotal trial on the horizon, Praxis has pulled in more money to fund its R&D activities. Eventide Asset Management led the series C1 round with support from fellow existing investors Vida Ventures, Novo Holdings, Blackstone Life Sciences and OCV Partners. Praxis also disclosed investment from nine first-time backers including Avoro Capital Advisors and Qatar Investment Authority.
The $110 million round will support development of PRAX-114, which is in phase 2 development in major depressive disorder (MDD) and perimenopausal depression. Praxis is testing oral formulations of the GABAA positive allosteric modulator, making its approach similar to Sage Therapeutics’ stuttering effort to bring SAGE-217 to market. SAGE-217 failed a phase 3 trial last year, leading Sage to propose starting three new trials in postpartum depression and subsets of MDD patients.
Praxis’ second clinical candidate, PRAX-944, is a T-type calcium channel blocker in development as a treatment for essential tremor. That therapeutic idea has attracted other companies. Last year, Jazz Pharmaceuticals bought Cavion for T-type calcium channel modulator CX-8998. In May, Neurocrine Biosciences took up its option on Idorsia’s T-type calcium channel blocker ACT-709478.
The two lead assets are trailed by PRAX-562, a phase 1 treatment for genetic epilepsies and pain, and another two treatments for genetic epilepsies that are yet to reach the clinic. The indications targeted by the pipeline reflect Praxis’ belief that it can use new understanding about the genetic causes of epilepsy to develop treatments for diseases driven by the same brain circuits.
그리고 3개월 후에 Nasdaq IPO를 했습니다. 가격과 주식수가 늘어서 결국 $190 Million이나 되는 큰 규모를 할 수 있었습니다. 전광석화같이 보이지만 2020년 이전의 활동이 비공개여서 그 준비기간이 매우 잘 되었다고 생각할 수 있습니다.
Praxis clinches $190M IPO, surpassing even its upsized target – 10/15/2020
2020 is turning out to be a big year for Praxis Precision Medicines. After launching in May with $100 million, the CNS-focused biotech wasted no time topping up its coffers with another $110 million. Now, it’s pulled off a $190 million IPO, upgrading from the $100 million listing it originally sought in September and the revised goal of $175 it set on Thursday.
The old plan was to sell 7.4 million shares at a price between $16 and $18 apiece, but the company bumped the offering up to 10 million shares at $17 to $18 each. Praxis ended up selling the 10 million shares at $19 apiece.
As its name suggests, Praxis is taking aim at the genetic underpinnings of brain disorders to create precision medicines for them. It has three programs in the clinic for depression, essential tremor, epilepsy and pain.
About $70 million to $80 million of the IPO proceeds will bankroll the phase 2a and phase 2/3 trials of lead asset PRAX-114, an oral modulator of GABAA receptors, according to a securities filing. The company is testing the drug in major depressive disorder and perimenopausal depression.
The company tagged $30 million to $40 million to see a second program, PRAX-944, through phase 2a and phase 2/3 studies in essential tremor. Another $20 million to $30 million will fund a phase 1 study in healthy people, as well as the first patient trial, for PRAX-562, a treatment for genetic epilepsies and pain disorders.
All told, the IPO proceeds, along with its previous funding rounds, should carry Praxis through the next 18 months, the company said in the filing.
IPO한지 1년 후 $91 Million 주식공모를 해서 현금을 늘렸습니다. 파이프라인은 여전히 PRAX-114, Ulixacaltamide (PRAX-944) 및 PRAX-562로 세개 프로그램이 진행 중이었습니다.
Praxis Precision Medicines Prices Public Offering of Common Stock – Globe Newswire 5/13/2021
Praxis Precision Medicines, Inc. (NASDAQ: PRAX), a clinical-stage biopharmaceutical company translating genetic insights into the development of therapies for central nervous system disorders (CNS) characterized by neuronal imbalance, today announced that it has priced an underwritten public offering of 5,000,000 shares of its common stock at a public offering price of $18.25 per share. The gross proceeds to Praxis from the offering are expected to be approximately $91.25 million, before deducting the underwriting discounts and commissions and other offering expenses. Praxis has granted the underwriters a 30-day option to purchase up to an additional 750,000 shares of its common stock.
All shares in the offering are to be sold by Praxis. The offering is expected to close on or about May 18, 2021, subject to satisfaction of customary closing conditions. Praxis intends to use the net proceeds from the offering, together with its existing cash, cash equivalents and short-term investments, to (i) advance PRAX-114 into and through the completion of the Phase 2/3 Aria Study in monotherapy major depressive disorder (“MDD”), which is intended to satisfy one of two registrational trials required by the U.S. Food and Drug Administration to support clinical efficacy for monotherapy MDD, advance PRAX-114 into and through the completion of Praxis’ Phase 2 trial for the adjunctive treatment of MDD, complete Part B (perimenopausal depression) of the Phase 2a clinical trial for PRAX-114, initiate a Phase 3 monotherapy trial in MDD, initiate and complete a Phase 2 trial in post-traumatic stress disorder (“PTSD”), initiate and complete a Phase 2 trial in essential tremor (“ET”) and pursue the development of PRAX-114 in an additional indication; (ii) complete its ongoing Phase 2a clinical trial and a Phase 2 randomized, controlled clinical trial for PRAX-944 in ET and initiate and complete a Phase 2 trial of PRAX-944 in Parkinson’s Disease; (iii) complete its ongoing PRAX-562 Phase 1 healthy volunteer trial and initiate and complete Phase 2 trials of PRAX-562 in Short-lasting Unilateral Neuralgiform headache attacks with Conjunctival injection and Tearing (“SUNCT”), Short-lasting Unilateral Neuralgiform headache with Autonomic symptoms (“SUNA”), and Trigeminal Neuralgia (“TN”), and in developmental and epileptic encephalopathies (“DEEs”), including SCN8A-DEE and SCN2A-DEE, and (iv) advance other programs in its pipeline and support working capital and other general corporate purposes.
BofA Securities, Cowen and Piper Sandler are acting as joint bookrunning managers for the offering, and Wedbush PacGrow is acting as lead manager for the offering.
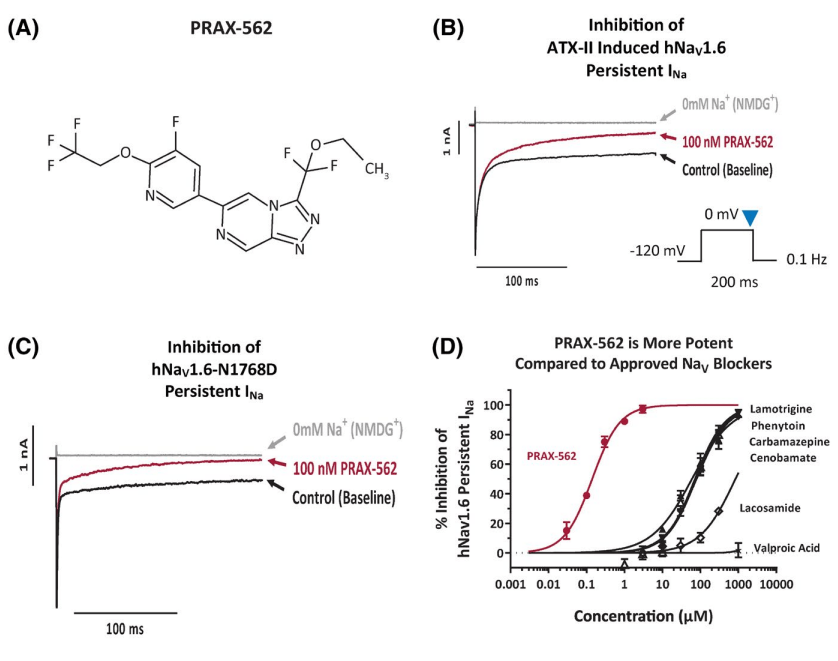
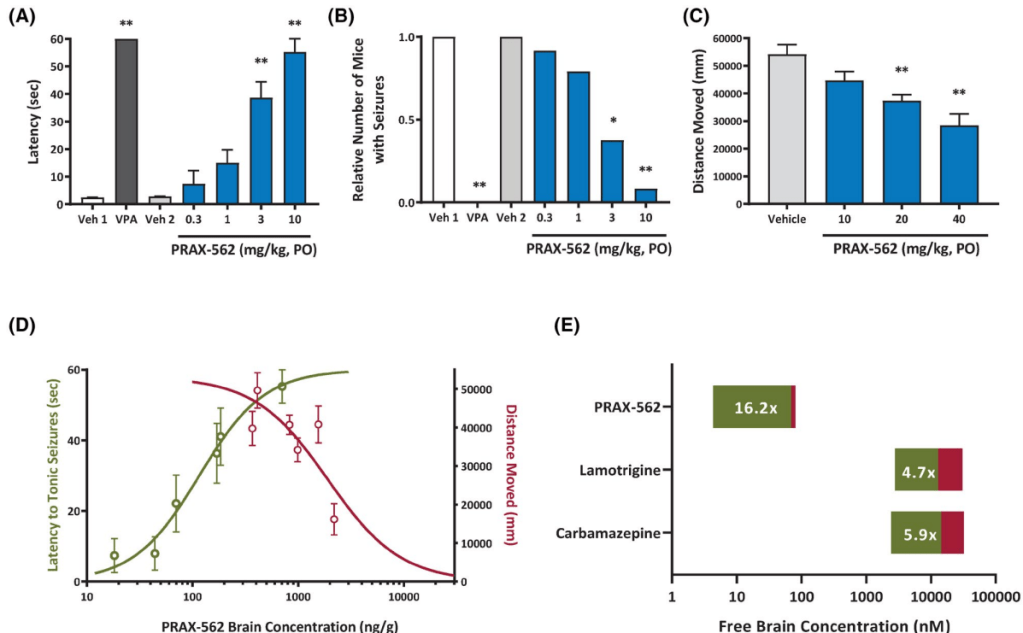
2022년 1월에 Epilepsia 논문에 PRAX-562의 전임상 연구결과를 발표하였습니다. 기존에 승인된 NaV blockers에 비해 월등히 우수한 약효 를 확인했습니다.
Sage의 Zuranolone에 대항하는 Praxis의 약물 PRAX-114는 primary end points, secondary end points 모두 실망스러운결과를 얻었습니다. 이로 인해 정리해고와 함께 PRAX-562와 PRAX-944 프로그램에 집중하는 것으로 전략이 신속히 수정되었습니다. 가장 선도하던 약물이 실패했을 때, 어떻게 회사를 Turnaround해야 하는지에 대해 잘 보여주는 사례입니다.
Praxis Precision Medicines’ challenge to Sage Therapeutics has unraveled. With a phase 2/3 clinical trial in major depressive disorder (MDD) failing comprehensively, Praxis is stopping work on a clutch of other studies, laying off staff and pinning its hopes on two other candidates.
The failed candidate, PRAX-114, is a type of nervous system drug known as an extrasynaptic GABAA receptor preferring positive allosteric modulator. Sage has gone some way toward validating the mechanism of action with its own drug zuranolone. Yet, Praxis’ bid to improve on the competition by providing a fast, durable antidepressant effect across MDD symptoms, a wider therapeutic window and simple nightly dosing has come unstuck.
Praxis is yet to share data from the phase 2/3 Aria trial, but everything that is known is bad. The study failed its primary endpoint, which looked at change in a depression score at Day 15, and missed all of its secondary endpoints.
“We are surprised and disappointed in the Aria study results,” Praxis CEO Marcio Souza said in a June 6 statement. “PRAX-114 was well-tolerated … yet the effect did not deliver to meet the needs of patients. Praxis is committed to our mission to help people suffering from CNS disorders and will prioritize our programs in movement disorders and epilepsy moving forward.”
The prioritization of the movement disorder and epilepsy programs will see Praxis lay off staff—it had 139 employees as of mid-February, up from 62 at the end of 2020—and stop work on other PRAX-114 trials. The biotech is also closing screening in its phase 2 Acapella study in MDD, positioning it to deliver results in the third quarter, stopping enrollment in a post-traumatic stress disorder phase 2 trial and discontinuing an essential tremor trial.
Through the changes, Praxis expects to extend its cash runway into 2024, giving it the time to rebuild its pipeline around its drugs PRAX-944 and PRAX-562. Top-line results from a phase 2b clinical trial of T-type calcium channel blocker PRAX-944 in the daytime treatment of essential tremor are due in the second half of the year, the same period given for epilepsy prospect PRAX-562 to enter phase 2 trials.
Praxis needs positive data on the candidates to drum up renewed enthusiasm for its pipeline. Shares in the biotech fell more than 60% to around $3 in premarket trading following news of the phase 2/3 flop.
위기는 기회를 동반한다고 하죠. PRAX-114의 실패, 정리해고와 PRAX-562의 Clinical Hold 후 다시 임상 재개 등 어려운 1년을 보낸데 대한 보상과 같은 UCB와 최대 $100 Million 규모의 공동연구계약을 맺습니다. KCNT-1 related epilepsy 프로그램으로 라이선싱 옵션이 있는 딜이었습니다.
It’s been a tough year of layoffs and pipeline culls for Praxis Precision Medicines, but the biotech is ending 2022 on a positive note courtesy of a collaboration with UCB to develop a potential first-ever treatment for a specific type of epilepsy.
The collaboration is based upon Praxis’ PRAX-020 program to discover small-molecule therapeutics as potential treatments of KCNT1-related epilepsies, for which there are currently no approved treatments. As well as an upfront payment from the Belgian biopharma, Praxis will be eligible for development and commercial milestone payments to the tune of up to $100 million, on top of royalties. UCB retains an exclusive option to in-license global development and commercialization rights to any KCNT1 small-molecule candidate that results from the agreement.
The money will come as welcome news to Praxis, which laid off staff and halted a clutch of clinical trials after its nervous system drug PRAX-114 failed to deliver in a phase 2 study in June. The company moved to prioritize its programs in movement disorders and epilepsy, a decision that appears to have paid off with the UCB deal.
Not that Praxis’ bank account is empty—the Boston-based company had $123.7 million in cash and equivalents as of the end September. It may be a drop of over $150 million on the funds the biotech entered the year with, but Praxis reckoned it was still enough to fund it through the start of 2024, even before any payments from UCB are taken into account.
“Our internal research efforts give us confidence that small molecules can selectively inhibit the KCNT1 channel and potentially could be an effective treatment for individuals suffering from KCNT1-related epilepsy,” Praxis CEO Marcio Souza said in a postmarket release Tuesday. “The collaboration with UCB validates this approach and will allow us to accelerate efforts toward a potential treatment for KCNT1 patients.”
As well as a number of other potential epilepsy programs in preclinical development, Praxis has one already in phase 2, with others due to enter phase 1 and 2 before the end of the year. One of these, PRA-562, was recently released from an FDA clinical hold.
Praxis의 Lead Program인 Ulixacaltamide (PRAX-944)의 임상3상 진입과 함께 $63 Million Public financing을 할 수 있었습니다.
Praxis Precision Medicines, Inc. (NASDAQ: PRAX), a clinical-stage biopharmaceutical company translating genetic insights into the development of therapies for central nervous system (CNS) disorders characterized by neuronal excitation-inhibition imbalance, today announced the closing of the underwritten public offering of shares of common stock and pre-funded warrants, including the full exercise of the underwriters’ overallotment option. The net proceeds from the offering are $63.3 million, after deducting underwriting discounts and commissions and estimated offering expenses. Together with the Company’s existing cash, cash equivalents and marketable securities, this extends the cash runway into the first quarter of 2025. The proceeds will be used to advance the development of ulixacaltamide into two Phase 3 studies for essential tremor, to continue clinical development of PRAX-562, PRAX-222 and PRAX-628 for various epilepsies, and for working capital and other general corporate purposes.
The Company has initiated a study evaluating PRAX-628 in photo-sensitive epilepsy patients, also known as a PPR study, with expected readout by year end. Similar studies have shown a positive correlation to anti-seizure medicines (ASMs) used to treat focal epilepsy. With this study, Praxis plans to validate the mechanism of action of PRAX-628 and inform the study design for its Phase 2b program.
Praxis remains on-track for reading out the Phase 2 Study for PRAX-562 and the first cohort for PRAX-222 in DEEs by year end.
“This financing provides the means to continue advancing our portfolio in movement disorders and in epilepsy closer to patients, with a number of catalysts this year,” said Marcio Souza, president and chief executive officer of Praxis. “We are excited to advance our Phase 3 program in essential tremor, while also initiating the PPR study to inform and de-risk our program in focal epilepsy. Additionally, we are pleased to have received support from both existing and new investors.”
About Praxis
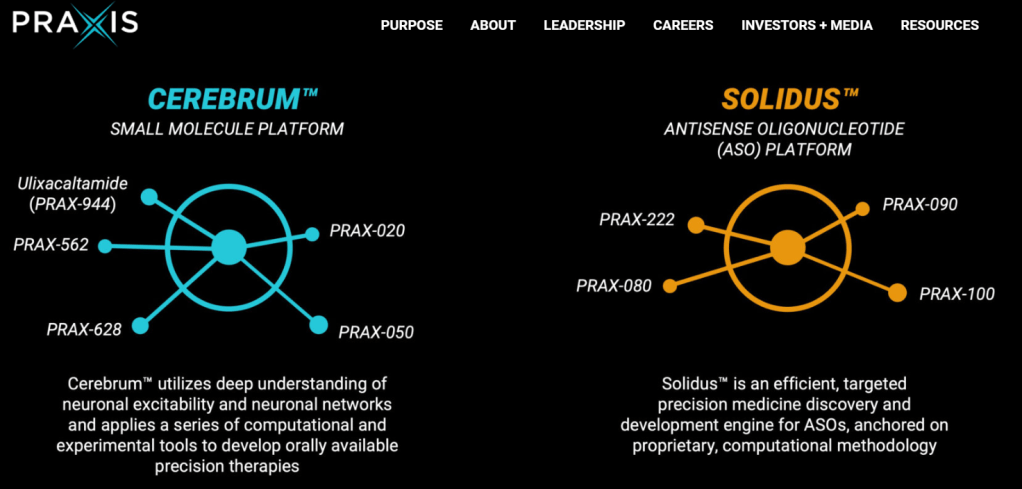
Praxis Precision Medicines is a clinical-stage biopharmaceutical company translating insights from genetic epilepsies into the development of therapies for CNS disorders characterized by neuronal excitation-inhibition imbalance. Praxis is applying genetic insights to the discovery and development of therapies for rare and more prevalent neurological disorders through our proprietary small molecule platform, Cerebrum™, and antisense oligonucleotide (ASO) platform, Solidus™, using our understanding of shared biological targets and circuits in the brain. Praxis has established a diversified, multimodal CNS portfolio including multiple programs across movement disorders and epilepsy, with four clinical-stage product candidates. For more information, please visit www.praxismedicines.com and follow us on Facebook, LinkedIn and Twitter.
2023년 8월에 ulixacaltamide (PRAX-944)의 임상2상 결과를 발표했고 결과는 좋았습니다. 이 결과는 MDD International Conference에서 발표했습니다.
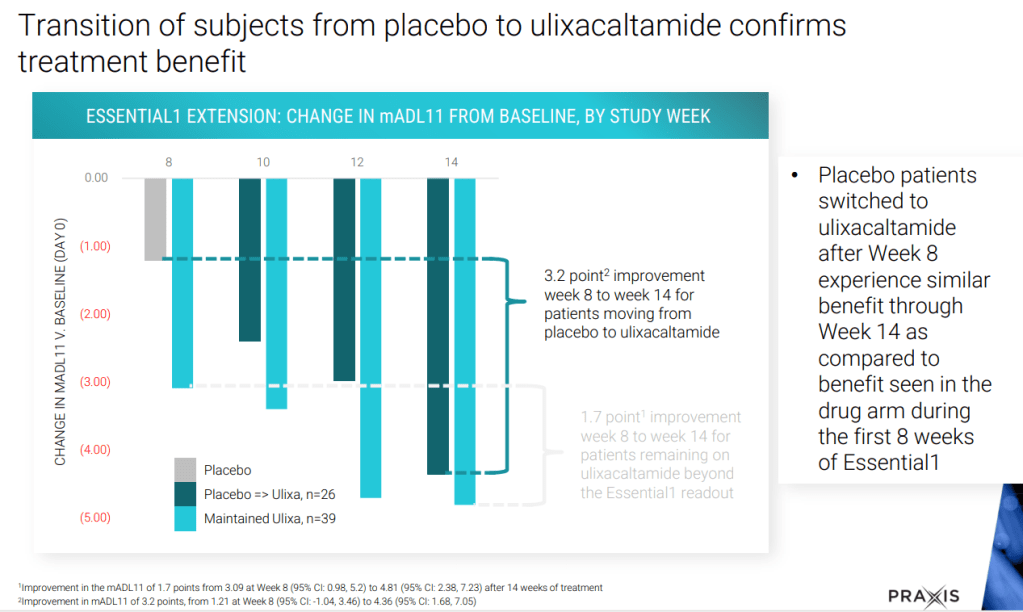
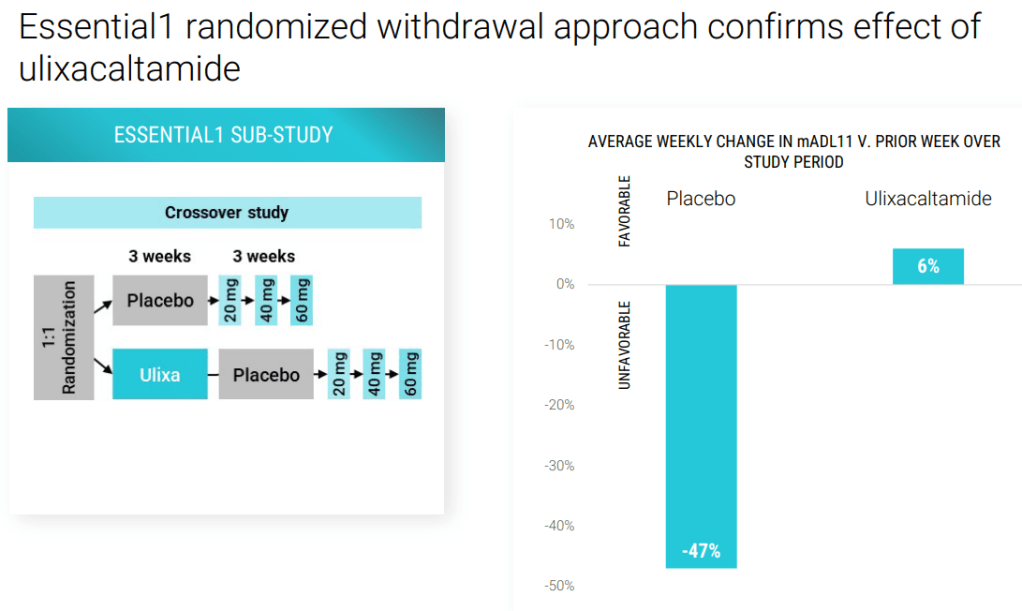
2023년 10월 Praxis R&D Day에서 파이프라인에 대한 총괄적인 발표를 했는데 그 자료는 아래에 링크합니다.
2024년 3월에 발표한 Corporate Presentation에 의하면 현재 파이프라인은 아래와 같습니다. Small Molecule Platform과 Antisense Oligonucleotide Platform으로 나뉘어져 있고 Ulixacaltamide (PRAX-944)가 임상3상으로 올해 하반기에 Pivotal result가 나올 예정입니다. 그 뒤를 이어서 PRAX-628과 PRAX-562가 임상2상에 진입해 있습니다. 최근 PRAX-629의 결과가 아주 좋게 나왔습니다.
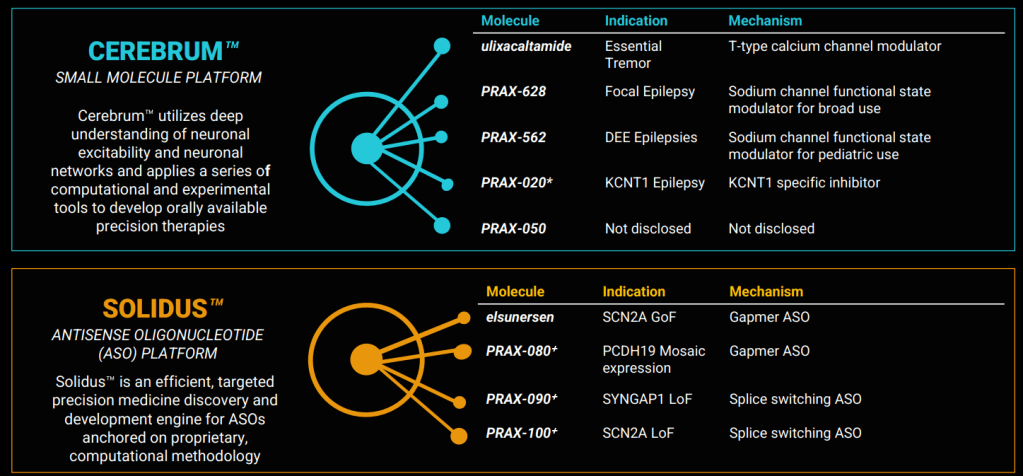
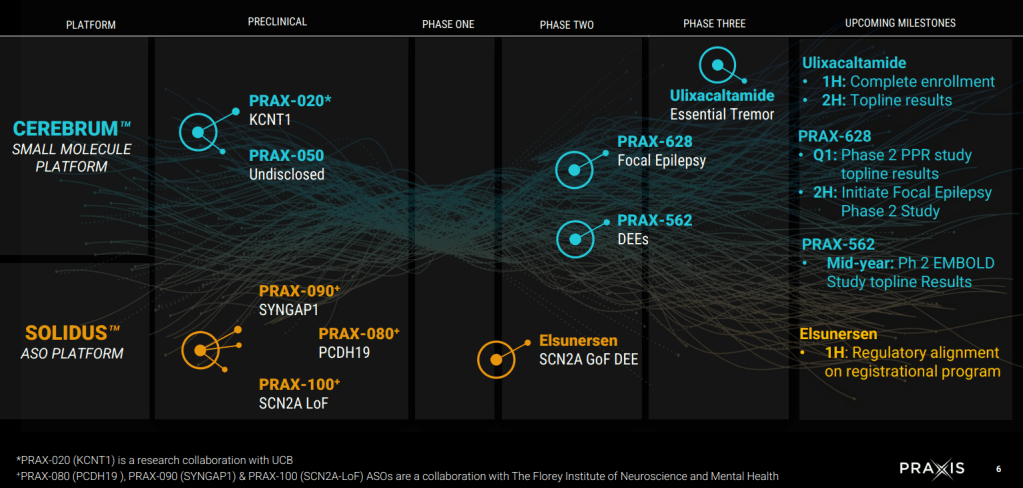
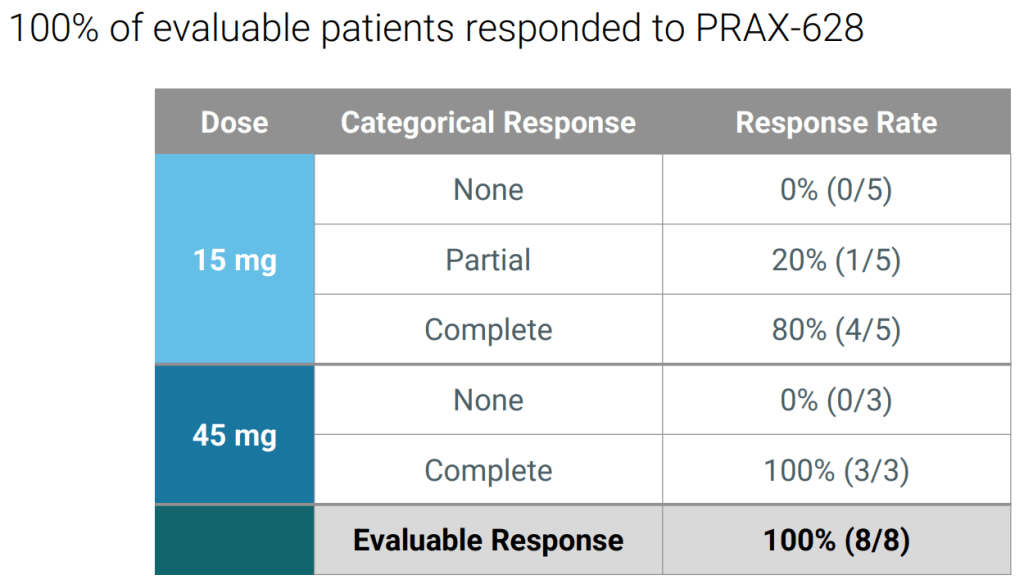
Praxis Precision Medicines has recorded a midphase win in epilepsy, linking the high dose of PRAX-628 to a 100% complete response rate to clear the path for a larger study in the second half of the year.
The phase 2a trial tested the sodium-channel drug candidate in epilepsy patients with photoparoxysmal response (PPR), a form of photosensitivity. Patients needed to have demonstrated PPR during screening to be evaluated in the trial. After treatment with PRAX-628, Praxis saw a 100% complete response rate in the high, 45mg cohort. The complete response rate on the lower dose was 80%.
Shares in Praxis rose 25% to almost $64 in the opening hours of trading on Tuesday from a Monday closing price of $50.58.
On a conference call with investors to discuss the results, Dan Friedman, M.D., professor of neurology at NYU Grossman School of Medicine, said the photoparoxysmal model is very useful for first-in-patient studies because “it has predictive ability for drugs that are ultimately efficacious in the clinic, especially those with broad spectrum activity.” Friedman cited cenobamate, brivaracetam and levetiracetam as molecules that suppressed PPR and were then shown to be efficacious.
Yet, it is unclear exactly what the effect on PPR means for the prospects of PRAX-628. Friedman said the field lacks “a really good relationship between the degree of suppression we see in these relatively small studies with a heterogeneous group of patients with a specific type of epilepsy, usually a generalized epilepsy, and ultimate efficacy in the clinic.”
45mg 환자들에서 8명중의 8명 (100%)의 Complete Response를 얻었다고 발표를 했습니다. 아직 환자수가 많지 않아서 계속 기대를 가지고 지켜볼 필요가 있습니다.


























!['무빙' 고윤정 "연기 칭찬, 행복해…처음으로 인정받아" [인터뷰]](https://orgthumb.mt.co.kr/06/2023/08/2023082509437220377_1.jpg) /
/!['무빙' 고윤정 "연기 칭찬, 행복해…처음으로 인정받아" [인터뷰]](https://orgthumb.mt.co.kr/06/2023/08/2023082509437220377_2.jpg)
!['무빙' 고윤정 "연기 칭찬, 행복해…처음으로 인정받아" [인터뷰]](https://orgthumb.mt.co.kr/06/2023/08/2023082509437220377_3.jpg)
!['무빙' 고윤정 "연기 칭찬, 행복해…처음으로 인정받아" [인터뷰]](https://orgthumb.mt.co.kr/06/2023/08/2023082509437220377_4.jpg)
!['무빙' 고윤정 "연기 칭찬, 행복해…처음으로 인정받아" [인터뷰]](https://orgthumb.mt.co.kr/06/2023/08/2023082509437220377_5.jpg)